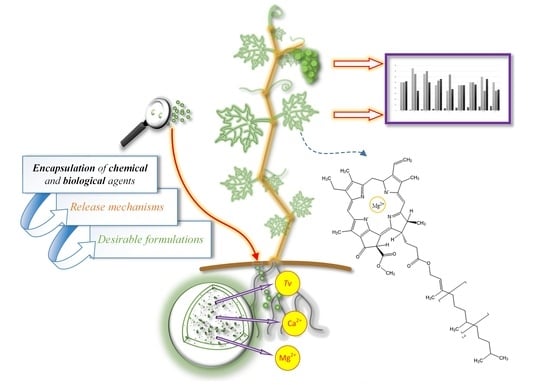
Tailoring Alginate/Chitosan Microparticles Loaded with Chemical and Biological Agents for Agricultural Application and Production of Value-Added Foods
Abstract
:1. Introduction
2. Biopolymeric Microparticles—Carrier System Composed of Alginate and Chitosan
3. A Cost-Efficient Method to Produce Microparticles—Ionic Gelation
4. Conventional Formulations vs. Advanced Formulations—A Necessity for Advanced Carrier Systems
5. Plant Metabolites (Biologically Active Compounds) and Their Importance in Functional Food Production
6. Significance of Chemical and Biological Agents Encapsulation in Sustainable Agriculture
7. Guidelines on Developing Biopolymeric Microparticles
7.1. Effect of Chemical Agents on the Growth and Viability of Biological Agent T. viride
7.2. Recommended Guidelines to Prepare Microparticles Loaded with Chemical and Biological Agents
7.3. Characterization of Microparticles Loaded with Chemical and Biological Agents
7.3.1. Morphological Characteristics—Microscopical Observations
7.3.2. Molecular Interactions between Components in Microparticles
7.3.3. Encapsulation Efficiency, Loading Capacity, and Swelling Degree
7.3.4. In Vitro Release of Chemical and Biological Agents
8. Application of Biopolymeric Microparticles and Proposed Guidelines
9. Influence of Microparticles Treatments on the Synthesis of Plant Metabolites
10. Application of Microparticles in the Cultivation of Other Plants—Unpublished Data
11. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Lenssena, K.G.M.; Bast, A.; de Boera, A. Clarifying the health claim assessment procedure of EFSA will benefit functional food innovation. J. Funct. Foods 2018, 47, 386–396. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 20, 309. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.; Lattanzio, V.M.T.; Angela Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. ISBN 81-308-0034-9. [Google Scholar]
- Mampholo, B.M.; Sivakumar, D.; Beukes, M.; van Rensburg, W.J. Effect of modified atmosphere packaging on the quality and bioactive compounds of Chinese cabbage (Brassica rapa L. ssp. chinensis). J. Sci. Food Agric. 2013, 93, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Anand, S. Various approaches for the secondary metabolite production through plant tissue culture. Pharmacia 2010, 1, 1–7. [Google Scholar]
- Banerjee, M.R.; Yesmin, L.; Vessey, J.K. Plant-Growth-Promoting Rhizobacteria as Biofertilizers and Biopesticides. In Handbook of Microbial Biofertilizers; Rai, M., Ed.; Food Products Press: New York, NY, USA, 2006; pp. 140–141. ISBN 9781560222705. [Google Scholar]
- Avioa, L.; Sbrana, C.; Giovannetti, M.; Frassin, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Bjorkman, T.; Harman, G.E. Solubilization of phosphate and micronutrients by the plant-growth promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.L.; Bueno, L.A. Development of Trichoderma sp. formulations in encapsulated granules (CG) and evaluation of conidia shelf-life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- White, P.J. Calcium channels in higher plants. Biochim. Biophys. Acta Biomembr. 2000, 1465, 171–189. [Google Scholar] [CrossRef] [Green Version]
- El-Beltagi, H.S.; Mohamed, H.I. Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Not. Bot. Horti. Agrobot. Cluj-Napoca 2013, 41, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, D. Exogenous calcium alleviates the impact of cadmium induced oxidative stress in Lens culinaris Medic. seedlings through modulation of antioxidant enzyme activities. J. Crop Sci. Biotechnol. 2012, 15, 325–334. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; AbdAllah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Peng, H.; Yang, T.; Whitaker, B.; Huang, L.; Sun, J.; Chen, P. Effect of calcium on strawberry fruit flavonoid pathway gene expression and anthocyanin accumulation. Plant Physiol. Biochem. 2014, 82, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef] [Green Version]
- Siddique, M.H.; Al-Whaibi, M.H.; Sakran, A.H.; Basalah, M.O.; Ali, H.M. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int. J. Mol. Sci. 2012, 13, 6604–6619. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhao, C.; Zhang, Y.; Wang, X.; Wang, X.; Wang, J.; Wang, F.; Bi, Y. Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis seedlings. Protoplasma 2016, 253, 185–200. [Google Scholar] [CrossRef]
- Fujita, Y.; Hara, Y.; Suga, C.; Morimoto, T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. II. A new medium for the production of shikonin derivatives. Plant Cell Rep. 1981, 1, 61–63. [Google Scholar] [CrossRef]
- Ohlsson, A.B.; Berglund, T. Effect of high MnSO4 levels on cardenolide accumulation by Digitalis lanata tissue cultures in light and darkness. J. Plant Physiol. 1989, 135, 505–507. [Google Scholar] [CrossRef]
- Trejo-Tapia, G.; Jimenez-Aparicio, A.; Rodriguez-Monroy, M.; De Jesus-Sanchez, A.; Gutierrez-Lopez, G. Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tissue Organ. Cult. 2001, 67, 19–23. [Google Scholar] [CrossRef]
- Mullins, M.G.; Bouquet, A.; Williams, L.E. Biology of the Grapevine; Cambridge University Press: New York, NY, USA, 1992; ISBN 978-0521305075. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, Hormones and Plant Regulators: Auxins and Gibberellins, 4th ed.; Wadsworth Publishing Company: Belmont, CA, USA, 1992; ISBN 10: 0534983901. [Google Scholar]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Narula, A.; Kumar, S.; Srivastava, P.S. Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep. 2005, 24, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, R.; Lowenberg-Deboer, J. Precision Agriculture and Sustainability. Precis. Agric. 2004, 5, 359–387. [Google Scholar] [CrossRef]
- Kudasova, D.; Mutaliyeva, B.; Vlahoviček-Kahlina, K.; Jurić, S.; Marijan, M.; Khalus, S.; Prosyanik, A.; Šegota, S.; Španić, N.; Vinceković, M. Encapsulation of Synthesized Plant Growth Regulator Based on Copper(II) Complex in Chitosan/Alginate Microcapsules. Int. J. Mol. Sci. 2021, 22, 2663. [Google Scholar] [CrossRef] [PubMed]
- Vlahoviček-Kahlina, K.; Jurić, S.; Marijan, M.; Mutaliyeva, B.; Khalus, S.; Prosyanik, A.; Vinceković, M. Synthesis, Characterization, and Encapsulation of Novel Plant Growth Regulators (PGRs) in Biopolymer Matrices. Int. J. Mol. Sci. 2021, 22, 1847. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Kulshreshtha, N.M.; Dixit, M.; Jadhav, I.; Shrivastava, D.; Bisen, P.S. Nanodentistry: Novel approaches. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 751–776. [Google Scholar]
- Singh, M.; Hemant, K.; Ram, M.; Shivakumar, H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Haffner, F.B.; Diab, R.; Pasc, A. Encapsulation of probiotics: Insights into academic and industrial approaches. AIMS Mater. Sci. 2016, 3, 114–136. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.; Vinceković, M.; Jurić, S.; Viskić, M.; Jambrak, A.R.; Donsì, F. Food-Grade Colloidal Systems for the Delivery of Essential Oils. Food Rev. Int. 2021, 37, 1–45. [Google Scholar] [CrossRef]
- Lai, W.-F.; Rogach, A.L. Hydrogel-Based Materials for Delivery of Herbal Medicines. ACS Appl. Mater. Interfaces 2017, 9, 11309–11320. [Google Scholar] [CrossRef]
- Chakravarthi, S.S.; Robinson, D.H. Biodegradable nanoparticles. In Pharmaceutical Manufacturing Handbook; Gad, S.C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 535–565. ISBN 978-0-470-25958-0. [Google Scholar]
- Sundar, S.; Kundu, J.; Kundu, S.C. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11, 014104. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.F.; Rintoul, L.; Fredericks, P.M.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Jurić, S.; Tanuwidjaja, I.; Fuka, M.M.; Vlahoviček-Kahlina, K.; Marijan, M.; Boras, A.; Kolić, N.U.; Vinceković, M. Encapsulation of two fermentation agents, Lactobacillus sakei and calcium ions in microspheres. Colloids Surf. B Biointerfaces 2021, 197, 111387. [Google Scholar] [CrossRef]
- Fuka, M.M.; Maksimovic, A.Z.; Hulak, N.; Kos, I.; Radovcic, N.M.; Juric, S.; Tanuwidjaja, I.; Karolyi, D.; Vincekovic, M. The survival rate and efficiency of non-encapsulated and encapsulated native starter cultures to improve the quality of artisanal game meat sausages. J. Food Sci. Technol. 2021, 58, 710–719. [Google Scholar] [CrossRef]
- Hudson, D.; Margaritis, A. Biopolymer nanoparticle production for controlled release of biopharmaceuticals. Crit. Rev. Biotechnol. 2012, 34, 161–179. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Jurić, S.; Đorđević, V.; Barišić, L.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Chemometric evaluation of binary mixtures of alginate and polysaccharide biopolymers as carriers for microencapsulation of green tea polyphenols. Int. J. Food Prop. 2017, 20, 1971–1986. [Google Scholar] [CrossRef] [Green Version]
- Vinceković, M.; Topolovec Pintarić, S.; Jurić, S.; Viskić, M.; Jalšenjak, N.; Bujan, M.; Đermić, E.; Žutić, I.; Fabek Uher, S. Release of Trichoderma viride spores from microcapsules simultaneously loaded with chemical and biological agents. Agric. Conspec. Sci. 2017, 82, 395–401. [Google Scholar]
- Smidsrød, O. Solution properties of alginate. Carbohydr. Res. 1970, 13, 359–372. [Google Scholar] [CrossRef]
- Smidsrød, O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O.; Högdahl, B.; Øye, H.A.; Rasmussen, S.E.; Sunde, E.; Sørensen, N.A. Selectivity of Some Anionic Polymers for Divalent Metal Ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Montanucci, P.; Terenzi, S.; Santi, C.; Pennoni, I.; Bini, V.; Pescara, T.; Basta, G.; Calafiore, R. Insights in Behavior of Variably Formulated Alginate-Based Microcapsules for Cell Transplantation. BioMed Res. Int. 2015, 2015, 965804. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, A.G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium−Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- De Vos, P.; Spasojevic, M.; De Haan, B.J.; Faas, M.M. The association between in vivo physicochemical changes and inflammatory responses against alginate based microcapsules. Biomaterials 2012, 33, 5552–5559. [Google Scholar] [CrossRef] [Green Version]
- Eiselt, P.; Yeh, J.; Latvala, R.K.; Shea, L.D.; Mooney, D.J. Porous carriers for biomedical applications based on alginate hydrogels. Biomater. 2000, 21, 1921–1927. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; Alvarez-Ramirez, J.; Vernon-Carter, E. Effect of the weight ratio of alginate-modified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Benrebah, F.; Prevost, D.; Yezza, A.; Tyagi, R. Agro-industrial waste materials and wastewater sludge for rhizobial inoculant production: A review. Bioresour. Technol. 2007, 98, 3535–3546. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; De-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Paula, A.R.; Carolino, A.T.; Paula, C.O.; Samuels, R.I. The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide Imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasites Vectors 2011, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.K.; Craig, K.A.; Covert, D.; Rennie, R.J.; Smith, R.S. Liquid Rhizobial Inoculants for Lentil and Field Pea. J. Prod. Agric. 1995, 8, 547–552. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S. Encapsulation of Bacillus salmalaya 139SI using double coating biopolymer technique. Lett. Appl. Microbiol. 2018, 68, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.D.; Brouwer, I.D.; Fitzgerald, M.A. Plant metabolomics and its potential application for human nutrition. Physiol. Plant 2007, 132, 162–175. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Scossa, F.; Brotman, Y.; Lima, F.D.A.E.; Willmitzer, L.; Nikoloski, Z.; Tohge, T.; Fernie, A.R. Genomics-based strategies for the use of natural variation in the improvement of crop metabolism. Plant Sci. 2016, 242, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.; Koliopoulos, G.; Giatropoulos, A.; Menkissoglu-Spiroudi, U. Plant secondary metabolites against arthropods of medical importance. Phytochem. Rev. 2019, 18, 1255–1275. [Google Scholar] [CrossRef]
- Živković, I.; Jurić, S.; Vinceković, M.; Galešić, M.; Marijan, M.; Vlahovićek-Kahlina, K.; Mikac, K.; Lemic, D. Polyphenol-Based Microencapsulated Extracts as Novel Green Insecticides for Sustainable Management of Polyphagous Brown Marmorated Stink Bug (Halyomorpha halys Stål, 1855). Sustainability 2020, 12, 10079. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 873–886. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dissanayake, A.A.; Zhang, C.-R.; Mills, G.L.; Nair, M.G. Cultivated maitake mushroom demonstrated functional food quality as determined by in vitro bioassays. J. Funct. Foods 2018, 44, 79–85. [Google Scholar] [CrossRef]
- Strzałka, K.; Kostecka-Gugała, A.; Latowski, D. Carotenoids and Environmental Stress in Plants: Significance of Carotenoid-Mediated Modulation of Membrane Physical Properties. Russ. J. Plant Physiol. 2003, 50, 168–173. [Google Scholar] [CrossRef]
- Kun, Y.; Lule, U.S.; Xiao-Lin, D. Lycopene: Its Properties and Relationship to Human Health. Food Rev. Int. 2006, 22, 309–333. [Google Scholar] [CrossRef]
- Kong, K.-W.; Khoo, H.-E.; Prasad, K.N.; Ismail, A.; Tan, C.-P.; Rajab, N.F. Revealing the Power of the Natural Red Pigment Lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and Tomato Byproducts. Human Health Benefits of Lycopene and Its Application to Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; McCann, S.; Grant, B.J.B.; Trevisan, M.; Muti, P.; Freudenheim, J.L. Lung Function in Relation to Intake of Carotenoids and Other Antioxidant Vitamins in a Population-based Study. Am. J. Epidemiol. 2002, 155, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Park, S.U. Current results on the potential health benefits of lutein. EXCLI J. 2016, 15, 308–314. [Google Scholar]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2020, 1–56. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Viera, I.; Roca, M. Chemistry in the Bioactivity of Chlorophylls: An Overview. Curr. Med. Chem. 2018, 24, 4515–4536. [Google Scholar] [CrossRef] [Green Version]
- Ferruzzi, M.; Bohm, V.; Courtney, P.; Schwartz, S. Antioxidant and Antimutagenic Activity of Dietary Chlorophyll Derivatives Determined by Radical Scavenging and Bacterial Reverse Mutagenesis Assays. J. Food Sci. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; Van Der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plösch, T.; Kuipers, F.; Elferink, R.P.J.O.; Rosing, H.; et al. Nonlinear partial differential equations and applications: The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vogel, J.; Jonker-Termont, D.S.; Van Lieshout, E.M.; Katan, M.B.; Van Der Meer, R. Green vegetables, red meat and colon cancer: Chlorophyll prevents the cytotoxic and hyperproliferative effects of haem in rat colon. Carcinogenesis 2004, 26, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Egner, P.A.; Wang, J.-B.; Zhu, Y.-R.; Zhang, B.-C.; Wu, Y.; Zhang, Q.-N.; Qian, G.-S.; Kuang, S.-Y.; Gange, S.J.; Jacobson, L.P.; et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef] [Green Version]
- Marawaha, R.K.; Bansal, D.; Kaur, S.; Trehan, A. Wheat grass juice reduces transfusion requirement in patients with thalassemia major: A pilot study. Indian Pediatr. 2004, 41, 716–720. [Google Scholar] [PubMed]
- Fahey, J.W.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Egner, P.A.; Kensler, T.W.; Talalay, P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis 2005, 26, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangcharoen, W.; Phimphilai, S. Chlorophyll and total phenolic contents, antioxidant activities and consumer acceptance test of processed grass drinks. J. Food Sci. Technol. 2016, 53, 4135–4140. [Google Scholar] [CrossRef]
- Kończak, I.; Zhang, W. Anthocyanins—More Than Nature’s Colours. J. Biomed. Biotechnol. 2004, 2004, 239–240. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Vicente, S.; Veiga, M.; Calhau, C.; Morais, R.M.; Pintado, M.E. DNA agarose gel electrophoresis for antioxidant analysis: Development of a quantitative approach for phenolic extracts. Food Chem. 2017, 233, 45–51. [Google Scholar] [CrossRef]
- Yan, S.; Shao, H.; Zhou, Z.; Wang, Q.; Zhao, L.; Yang, X. Non-extractable polyphenols of green tea and their antioxidant, anti-α-glucosidase capacity, and release during in vitro digestion. J. Funct. Foods 2018, 42, 129–136. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J.; Piwowar, A. Commercialization aspects of carotenoids. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–357. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechowski, Z.; Białczyk, J. Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biol. Plant. 1993, 35, 53–62. [Google Scholar] [CrossRef]
- Wallace, A.; Mueller, R.T.; Wood, R.A. Arsenic phytotoxicity and interactions in bush bean plants grown in solution culture. J. Plant Nutr. 1980, 2, 111–113. [Google Scholar] [CrossRef]
- Pal, R.; Laloraya, M. Effect of Calcium Levels on Chlorophyll Synthesis in Peanut and Linseed Plants. Biochem. Physiol. Pflanz. 1972, 163, 443–449. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop. Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Bilesky-José, N.; De Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef] [Green Version]
- Howell, C.R. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keswani, C.; Mishra, S.; Sarma, B.K.; Singh, S.P.; Singh, H.B. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol. Biotechnol. 2013, 98, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Ihrmark, K.; Asmail, N.; Ubhayasekera, W.; Melin, P.; Stenlid, J.; Karlsson, M. Comparative Molecular Evolution of Trichoderma Chitinases in Response to Mycoparasitic Interactions. Evol. Bioinform. 2010, 6, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Grondona, I.; Hermosa, R.; Tejada, M.; Gomis, M.D.; Mateos, P.F.; Bridge, P.D.; Monte, E.; Garcia-Acha, I. Physiological and biochemical characterization of Trichoderma harzianum, a biological control agent against soilborne fungal plant pathogens. Appl. Environ. Microbiol. 1997, 63, 3189–3198. [Google Scholar] [CrossRef] [Green Version]
- Jurić, S. Bioencapsulation as a Sustainable Delivery of Active Agents for Plant Nutrition/Protection and Production of Functional Foods. Doctoral Thesis, Faculty of Food Technology and Biotechnology, University of Zagreb, Zagreb, Croatia, 2020. [Google Scholar]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M.; Aly, M.D.; Lashin, S.M. Application of Fungicides Alternatives as Seed Treatment for Controlling Root Rot of Some Vegetables in Pot Experiments. Adv. Life Sci. 2012, 2, 57–64. [Google Scholar] [CrossRef]
- Lopes, A.R.D.O.; Locatelli, G.O.; Barbosa, R.D.M.; Junior, M.L.; Mascarin, G.M.; Finkler, C.L.L. Preparation, characterisation and cell viability of encapsulated Trichoderma asperellum in alginate beads. J. Microencapsul. 2020, 37, 270–282. [Google Scholar] [CrossRef]
- Lorenz, S.-C.; Humbert, P.; Wassermann, M.; Mackenstedt, U.; Patel, A.V. A broad approach to screening of Metarhizium spp. blastospores for the control of Ixodes ricinus nymphs. Biol. Control. 2020, 146, 104270. [Google Scholar] [CrossRef]
- Feng, J.; Dou, J.; Zhang, Y.; Wu, Z.; Yin, D.; Wu, W. Thermosensitive Hydrogel for Encapsulation and Controlled Release of Biocontrol Agents to Prevent Peanut Aflatoxin Contamination. Polymers 2020, 12, 547. [Google Scholar] [CrossRef] [Green Version]
- Mancera-López, M.E.; Izquierdo-Estévez, W.F.; Escalante-Sánchez, A.; Ibarra, J.E.; Barrera-Cortés, J. Encapsulation of Trichoderma harzianum conidia as a method of conidia preservation at room temperature and propagation in submerged culture. Biocontrol Sci. Technol. 2018, 29, 107–130. [Google Scholar] [CrossRef]
- Feng, J.; Dou, J.; Wu, Z.; Yin, D.; Wu, W. Controlled Release of Biological Control Agents for Preventing Aflatoxin Contamination from Starch–Alginate Beads. Molecules 2019, 24, 1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, P.; Vemmer, M.; Patel, A.V. Increased neem extract content enhances drying survival of co-encapsulated Saccharomyces cerevisiae and decreases relative release of azadirachtin. Biocontrol Sci. Technol. 2018, 28, 185–191. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Vidal, S.; Patel, A.V. Encapsulation of Metarhizium brunneum enhances endophytism in tomato plants. Biol. Control 2018, 116, 62–73. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Vidal, S.; Patel, A.V. Cellulase enhances endophytism of encapsulated Metarhizium brunneum in potato plants. Fungal Biol. 2018, 122, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Krell, V.; Jakobs-Schoenwandt, D.; Persicke, M.; Patel, A.V. Endogenous arabitol and mannitol improve shelf life of encapsulated Metarhizium brunneum. World J. Microbiol. Biotechnol. 2018, 34, 108. [Google Scholar] [CrossRef]
- Humbert, P.; Przyklenk, M.; Vemmer, M.; Patel, A.V. Calcium gluconate as cross-linker improves survival and shelf life of encapsulated and dried Metarhizium brunneum and Saccharomyces cerevisiae for the application as biological control agents. J. Microencapsul. 2016, 34, 47–56. [Google Scholar] [CrossRef]
- Vinceković, M.; Jurić, S.; Đermić, E.; Topolovec-Pintarić, S. Kinetics and Mechanisms of Chemical and Biological Agents Release from Biopolymeric Microcapsules. J. Agric. Food Chem. 2017, 65, 9608–9617. [Google Scholar] [CrossRef]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Jurić, S.; Šegota, S.; Vinceković, M. Influence of surface morphology and structure of alginate microparticles on the bioactive agents release behavior. Carbohydr. Polym. 2019, 218, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jalšenjak, N.; Topolovec-Pintarić, S.; Đermić, E.; Bujan, M.; Jurić, S. Encapsulation of Biological and Chemical Agents for Plant Nutrition and Protection: Chitosan/Alginate Microcapsules Loaded with Copper Cations and Trichoderma viride. J. Agric. Food Chem. 2016, 64, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Seidl-Seiboth, V. Self versus non-self: Fungal cell wall degradation in Trichoderma. Microbiology 2012, 158, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.-Y.; Song, X.-L.; Liang, J.-L.; Zheng, S.-H.; Lin, Y. Disinfection of airborne spores of Penicillium expansum in cold storage using continuous direct current corona discharge. Biosyst. Eng. 2012, 113, 112–119. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef]
- Singh, T.; Saikia, R.; Jana, T.; Arora, D.K. Hydrophobicity and surface electrostatic charge of conidia of the mycoparasitic Trichoderma species. Mycol. Prog. 2004, 3, 219–228. [Google Scholar] [CrossRef]
- Daly, M.M.; Knorr, D. Chitosan-Alginate Complex Coacervate Capsules: Effects of Calcium Chloride, Plasticizers, and Polyelectrolytes on Mechanical Stability. Basiotechnol. Prog. 1988, 4, 76–81. [Google Scholar] [CrossRef]
- Irmanida, B.; Devi, R.; Kusdiantoro, M.; Wahono Esthi, P. Leydig Cells Encapsulation with Alginate-Chitosan: Optimization of Microcapsule Formation. J. Encapsulation Adsorpt. Sci. 2012, 2, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Selimoglu, S.M.; Elibol, M. Alginate as an immobilization material for MAb production via encapsulated hybridoma cells. Crit. Rev. Biotechnol. 2010, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.R.; Lagoa, R. Copper Ions Binding in Cu-Alginate Gelation. J. Carbohydr. Chem. 2006, 25, 219–232. [Google Scholar] [CrossRef]
- Goosen, M.F.A.; O’Shea, G.M.; Sun, M.F. Microencapsulation of Living Tissue and Cells. U.S. Patent 4806355A, 16 June 1987. [Google Scholar]
- Gåserød, O.; Smidsrød, O.; Skjåk-Bræk, G. Microcapsules of alginate-chitosan. I. A quantitative study of the interaction between alginate and chitosan. Biomaterial 1998, 19, 1815–1825. [Google Scholar] [CrossRef]
- Mi, F.-L.; Sung, H.-W.; Shyu, S.-S. Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr. Polym. 2002, 48, 61–72. [Google Scholar] [CrossRef]
- Shi, J.; Alves, N.M.; Mano, J.F. Chitosan coated alginate beads containing poly(N-isopropylacrylamide) for dual-stimuli-responsive drug release. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 84, 595–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasseröd, O.; Sannes, A.; Skjåk-Bræk, G. Microcapsules of alginate–chitosan. II. A study of capsule stability and permeability. Biomaterail 1999, 20, 773–783. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from algae. In Polysaccharides and Polyamides in the Food Industry: Properties, Production, and Patents; Steinbüchel, A.S., Rhee, K., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–30. ISBN 978-3-527-31345-7. [Google Scholar]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Bespalova, A.Y.; Motuzova, G.V.; Marfenina, O.E. Secondary mobilization of heavy metals in polluted soils under microbial influence (model experiment). Develop. Soil Sci. 2002, 28, 187–193. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.-S.; Ha, B.-J.; Park, S.-N.; Lee, Y.-S. Studies on the pH-dependent swelling properties and morphologies of chitosan/calcium-alginate complexed beads. Macromol. Chem. Phys. 1997, 198, 2971–2976. [Google Scholar] [CrossRef]
- Rajendran, A.; Basu, S. Alginate-Chitosan Particulate System for Sustained Release of Nimodipine. Trop. J. Pharm. Res. 2009, 8, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xue, W.; Liu, Q.; Yu, W.; Fu, Y.; Xiong, X.; Ma, X.; Yuan, Q. Swelling behaviour of alginate–chitosan microcapsules prepared by external gelation or internal gelation technology. Carbohydr. Polym. 2004, 56, 459–464. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A quantitative analysis of alginate swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Bajpai, S.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Hunkeler, D. Alginate−Oligochitosan Microcapsules. II. Control of Mechanical Resistance and Permeability of the Membrane. Chem. Mater. 2000, 12, 206–212. [Google Scholar] [CrossRef]
- Rokstad, A.M.A.; Lacík, I.; De Vos, P.; Strand, B.L. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv. Drug Deliv. Rev. 2014, 67–68, 111–130. [Google Scholar] [CrossRef]
- Roger, S.; Talbot, D.; Bee, A. Preparation and effect of Ca2+ on water solubility, particle release and swelling properties of magnetic alginate films. J. Magn. Magn. Mater. 2006, 305, 221–227. [Google Scholar] [CrossRef]
- Loh, Q.L.; Wong, Y.Y.; Choong, C. Combinatorial effect of different alginate compositions, polycations, and gelling ions on microcapsule properties. Colloid Polym. Sci. 2012, 290, 619–629. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of plant growth promoting Rhizobacteria—prospects and potential in agricultural sector: A review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Vinceković, M.; Bandić, L.M.; Jurić, S.; Jalšenjak, N.; Čaić, A.; Živičnjak, I.; Đermić, E.; Karoglan, M.; Osrečak, M.; Topolovec-Pintarić, S. The enhancement of bioactive potential in Vitis vinifera leaves by application of microspheres loaded with biological and chemical agents. J. Plant Nutr. 2019, 42, 543–558. [Google Scholar] [CrossRef]
- Jurić, S.; Stracenski, K.S.; Król-Kilińska, Ż.; Žutić, I.; Uher, S.F.; Đermić, E.; Topolovec-Pintarić, S.; Vinceković, M. The enhancement of plant secondary metabolites content in Lactuca sativa L. by encapsulated bioactive agents. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Ratajska, M.; Boryniec, S. Biodegradation of some natural polymers in blends with polyolefines. Polym. Adv. Technol. 1999, 10, 625–633. [Google Scholar] [CrossRef]
- Young, P.R.; Lashbrooke, J.G.; Alexandersson, E.; Jacobson, D.; Moser, C.; Velasco, R.; Vivier, M. The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genom. 2012, 13, 243. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, P.; Pérez, L.M. Calcium ions promote the response of Citrus limon against fungal elicitors or wounding. Phytochemistry 1996, 42, 595–598. [Google Scholar] [CrossRef]
- Penel, C.; Van Cutsem, P.; Greppin, H. Interactions of a plant peroxidase with oligogalacturonides in the presence of calcium ions. Phytochemistry 1999, 51, 193–198. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics–the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: The missing link in functional genomics strategies. Plant Cell 2002, 14, 1437–1440. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.D. Plant metabolomics: From holistic hope, to hype, to hot topic. New Phytol. 2006, 169, 453–468. [Google Scholar] [CrossRef]
- Heyman, H.M.; Dubery, I.A. The potential of mass spectrometry imaging in plant metabolomics: A review. Phytochem. Rev. 2016, 15, 297–316. [Google Scholar] [CrossRef]
- Jurić, S.; Vlahoviček-Kahlina, K.; Duralija, B.; Maslov Bandić, L.; Nekić, P.; Vinceković, M. Stimulation of plant secondary metabolites synthesis in soilless cultivated strawberries (Fragaria × ananassa Duchesne) using zinc- alginate microparticles. Turk. J. Agric. For. 2021, in press. [Google Scholar] [CrossRef]








| Biological Agent | Particle Type | Encapsulation Method/Material | Storage | Results | Application/Purpose | Literature |
|---|---|---|---|---|---|---|
| Trichoderma asperellum BRM-29104 conidia and microsclerotia |
|
|
|
|
| [120] |
| Metarhizium brunneum Ca8II, Cb15III, Cb16III and Cb16IV blastospores and Metarhizium pemphigi X1c blastospores |
|
|
|
|
| [121] |
| Aspergillus flavus H4-5 spores |
|
|
|
|
| [122] |
| Trichoderma harzianum CDBB-H1-125 conidia |
|
|
|
|
| [123] |
| Aspergillus flavus H4-5 spores |
|
|
|
|
| [124] |
| Saccharomyces cerevisiae Meyen ex E.C. Hansen |
|
|
|
|
| [125] |
| Metarhizium brunneum strain BIPESCO5 mycelium |
|
|
|
|
| [126] |
| Metarhizium brunneum CB15 mycelium |
|
|
|
|
| [127] |
| Metarhizium brunneum BIPESCO5 mycelium |
|
|
|
|
| [128] |
| Metarhizium brunneum ART2825 aero conidia and Saccharomyces cerevisiae H205 |
|
|
|
|
| [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurić, S.; Jurić, M.; Režek Jambrak, A.; Vinceković, M. Tailoring Alginate/Chitosan Microparticles Loaded with Chemical and Biological Agents for Agricultural Application and Production of Value-Added Foods. Appl. Sci. 2021, 11, 4061. https://0-doi-org.brum.beds.ac.uk/10.3390/app11094061
Jurić S, Jurić M, Režek Jambrak A, Vinceković M. Tailoring Alginate/Chitosan Microparticles Loaded with Chemical and Biological Agents for Agricultural Application and Production of Value-Added Foods. Applied Sciences. 2021; 11(9):4061. https://0-doi-org.brum.beds.ac.uk/10.3390/app11094061
Chicago/Turabian StyleJurić, Slaven, Marina Jurić, Anet Režek Jambrak, and Marko Vinceković. 2021. "Tailoring Alginate/Chitosan Microparticles Loaded with Chemical and Biological Agents for Agricultural Application and Production of Value-Added Foods" Applied Sciences 11, no. 9: 4061. https://0-doi-org.brum.beds.ac.uk/10.3390/app11094061