Effect of RhOx/CeO2 Calcination on Metal-Support Interaction and Catalytic Activity for N2O Decomposition
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalysts Characterization
2.3. N2O Decomposition Tests
3. Results and Discussion
3.1. TG-MS Study of Metal Precursors Decomposition

3.2. X-ray Diffraction, Raman Spectroscopy and N2 Adsorption at −196 °C Characterization


| Sample | Lattice Parameter (nm) | Crystal Size by Scherrer (nm) | Crystal Size by Williamson-Hall (nm) | BET Surface Area (m2/g) |
|---|---|---|---|---|
| Rh25/Ce25 | 0.5401 | 12 | 14 | 64 |
| Rh250/Ce25 | 0.5412 | 12 | 13 | 66 |
| Rh350/Ce25 | 0.5401 | 12 | 13 | 66 |
| Rh350/Ce250 | 0.5424 | 12 | 13 | 69 |
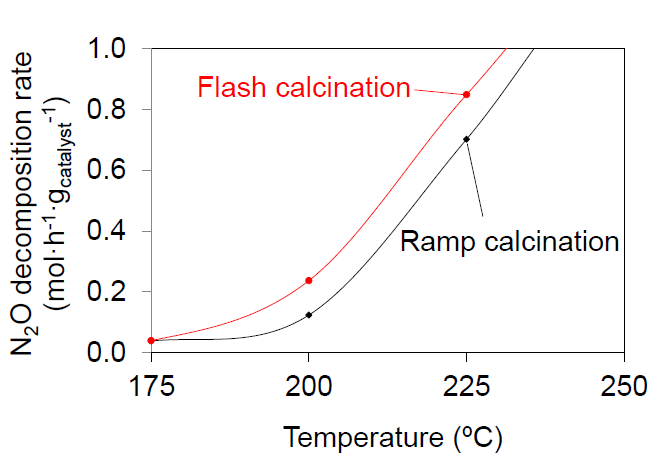
3.3. Catalytic Tests


3.4. Characterization by XPS of Fresh Catalysts and after in Situ Pre-Treatments with N2O at 225 °C

3.5. H2-TPR Characterization

3.6. TEM Characterization

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trovarelli, A.; de Leitenburg, C.; Boaro, M.; Dolcetti, G. The utilization of ceria in industrial catalysis. Catal. Today 1999, 50, 353–367. [Google Scholar]
- Luo, M.F.; Ma, J.M.; Lu, J.Q.; Song, Y.P.; Wang, Y.J. High-surface area CuO-CeO2 catalysts prepared by a surfactant-templated method for low-temperature CO oxidation. J. Catal. 2007, 246, 52–59. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Konsolakis, M.; Drosou, C.; Yentekakis, I.V. Support mediated promotional effects of Rare Earth Oxides (CeO2 and La2O3) on N2O decomposition and N2O reduction by CO or C3H6 over Pt/Al2O3 structured catalysts. Appl. Catal. B 2012, 123, 405–413. [Google Scholar] [CrossRef]
- Konsolakis, M.; Aligizou, F.; Goula, G.; Yentekakis, I.V. N2O decomposition over doubly-promoted Pt(K)/Al2O3-(CeO2-La2O3) structured catalysts: On the combined effects of promotion and feed composition. Chem. Eng. J. 2013, 230, 286–295. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.J.; Cauqui, M.A.; Gatica, J.M.; Larese, C.; Pérez Omil, J.A.; Pintado, J.M. Some recent results on metal/support interaction effects in NM/CeO2 (NM, noble metal) catalysts. Catal. Today 1999, 50, 175–206. [Google Scholar] [CrossRef]
- Fornasiero, P.; Kaspar, J.; Sergo, V.; Graziani, M. Redox Behavior of High-Surface-Area Rh-, Pt-, and Pd-Loaded Ce0.5Zr0.5O2 Mixed Oxide. J. Catal. 1999, 182, 56–69. [Google Scholar]
- Fornasiero, P.; Dimonte, R.; Rao, G.R.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-Loaded CeO2-ZrO2 Solid-Solutions as Highly Efficient Oxygen Exchangers, Dependence of the Reduction Behavior and the Oxygen Storage Capacity of the Structural-Properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Rao, G.; Mishra, B. Structural, redox and catalytic chemistry of ceria based materials. Bull. Catal. Soc. India 2003, 2, 122–134. [Google Scholar]
- Ivanova, A.S. Physicochemical and Catalytic Properties of Systems Based on CeO2. Kinet. Catal. 2009, 50, 797–815. [Google Scholar] [CrossRef]
- Miyazawa, T.; Okumura, K.; Kunimori, K.; Tomishige, K. Promotion of Oxidation and Reduction of Rh Species by Interaction of Rh and CeO2 over Rh/CeO2/SiO2. J. Phys. Chem. C 2008, 112, 2574–2583. [Google Scholar] [CrossRef]
- Vidmar, P.; Fornasiero, P.; Kaspar, J.; Gubitosa, G.; Graziani, M. Effects of Trivalent Dopants on the Redox Properties of Ce0.6Zr0.4O2 Mixed Oxide. J. Catal. 1997, 171, 160–168. [Google Scholar]
- Bera, P.; Gayen, A.; Hegde, M.S.; Lalla, N.P.; Spadaro, L.; Frusteri, F.; Arena, F. Promoting Effect of CeO2 in Combustion Synthesized Pt/CeO2 Catalyst for CO Oxidation. J. Phys. Chem. B 2003, 107, 6122–6130. [Google Scholar] [CrossRef]
- He, Q.; Mukerjee, S.; Shyam, B.; Ramaker, D.; Parres-Esclapez, S.; Illán-Gómez, M.J.; Bueno-López, A. Promoting effect of CeO2 in the electrocatalytic activity of rhodium for ethanol electro-oxidation. J. Power Sour. 2009, 193, 408–415. [Google Scholar] [CrossRef]
- Costa, L.O.O.; Vasconcelos, S.M.R.; Pinto, A.L.; Silva, A.M.; Mattos, L.V.; Noronha, F.B.; Borges, L.E.P. Rh/CeO2 catalyst preparation and characterization for hydrogen production from ethanol partial oxidation. J. Mater. Sci. 2008, 43, 440–449. [Google Scholar] [CrossRef]
- Kaspar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Parres-Esclapez, S.; Illán-Gómez, M.J.; Salinas-Martinez de Lecea, C.; Bueno-López, A. On the importance of the catalyst redox properties in the N2O decomposition over alumina and ceria supported Rh, Pd and Pt. Appl. Catal. B 2010, 96, 370–378. [Google Scholar] [CrossRef]
- Rico-Perez, V.; Parres-Esclapez, S.; Illan-Gomez, M.J.; Salinas-Martinez de Lecea, C.; Bueno-Lopez, A. Preparation, characterisation and N2O decomposition activity of honeycomb monolith-supported Rh/Ce0.9Pr0.1O2 catalysts. Appl. Catal. B 2011, 107, 18–25. [Google Scholar] [CrossRef]
- Rico-Pérez, V.; Velasco Beltrán, M.A.; He, Q.; Wang, Q.; Salinas-Martínez de Lecea, C.; Bueno-López, A. Preparation of ceria-supported rhodium oxide sub-nanoparticles with improved catalytic activity for CO oxidation. Catal. Commun. 2013, 33, 47–50. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Parres-Esclapez, S.; Lozano-Castelló, D.; Bueno-López, A. Relationship between surface area and crystal size of pure and doped cerium oxides. J. Rare Earths 2010, 28, 647–653. [Google Scholar] [CrossRef]
- Parres-Esclapez, S.; Such-Basañez, I.; Illán-Gómez, M.J.; Salinas-Martínez de Lecea, C.; Bueno-López, A. Study by isotopic gases and in situ spectroscopies (DRIFTS, XPS and Raman) of the N2O decomposition mechanism on Rh/CeO2 and Rh/χ-Al2O3 catalysts. J. Catal. 2010, 276, 390–401. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Atribak, I.; Bueno-López, A.; García-García, A. Influence of the cerium precursor on the physico-chemical features and NO to NO2 oxidation activity of ceria and ceria-zirconia catalysts. J. Mol. Catal. A Chem. 2010, 323, 52–58. [Google Scholar] [CrossRef]
- Terribile, D.; Trovarelli, A.; Llorca, J.; de Leitenburg, C.; Dolcetti, G. The preparation of high surface area CeO2-ZrO2 mixed oxides by a surfactant-assisted approach. Catal. Today 1998, 43, 79–88. [Google Scholar] [CrossRef]
- Mineshige, A.; Taji, T.; Muroi, Y.; Kobune, M.; Fujii, S.; Nishi, N.; Inaba, M.; Ogumi, Z. Oxygen chemical potential variation in ceria-based solid oxide fuel cells determined by Raman spectroscopy. Solid State Ionics 2000, 135, 481–485. [Google Scholar] [CrossRef]
- Bueno-Lopez, A.; Krishna, K.; Makkee, M.; Moulijn, J.A. Enhanced soot oxidation by lattice oxygen via La3+-doped CeO2. J. Catal. 2005, 230, 237–248. [Google Scholar] [CrossRef]
- Music, S.; Saric, A.; Popovic, S.; Ivanda, M. Formation and characterisation of nanosize α-Rh2O3 particles. J. Mol. Struct. 2009, 924, 221–224. [Google Scholar] [CrossRef]
- Pushkarev, V.V.; Kovalchuk, V.I.; d’Itri, J.L. Probing Defect Sites on the CeO2 Surface with Dioxygen. J. Phys. Chem. B 2004, 108, 5341–5348. [Google Scholar] [CrossRef]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-induced Raman spectra in doped CeO2. Phys. Rev. B 1994, 50, 13297–13307. [Google Scholar] [CrossRef]
- Bueno-López, A.; Such-Basáñez, I.; Salinas-Martínez de Lecea, C. Stabilization of active Rh2O3 species for catalytic decomposition of N2O on La-, Pr-doped CeO2. J. Catal. 2006, 244, 102–112. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Improved Metal-Support Interaction in Pt/CeO2-SiO2 Catalysts after Zinc Addition. J. Catal. 2002, 210, 127–136. [Google Scholar] [CrossRef]
- Zotin, F.M.Z.; Tournayan, L.; Varloud, J.; Perrichon, V.; Frety, R. Temperature-programmed reduction, limitation of the technique for determining the extent of reduction of either pure ceria or ceria modified by additives. Appl. Catal. A 1993, 98, 99–114. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Catalytic Science Series; Imperial College Press: London, UK, 2002; Volume 2, pp. 51–83. [Google Scholar]
- Bernal, S.; Calvino, J.J.; Cifredo, G.A.; Gatica, J.M.; Perez Omil, J.A.; Pintado, J.M. Hydrogen Chemisorption on Ceria, Influence of the Oxide Surface Area and Degree of Reduction. J. Chem. Soc. Faraday Trans. 1993, 89, 3499–3505. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rico-Pérez, V.; Bueno-López, A. Effect of RhOx/CeO2 Calcination on Metal-Support Interaction and Catalytic Activity for N2O Decomposition. Appl. Sci. 2014, 4, 468-481. https://0-doi-org.brum.beds.ac.uk/10.3390/app4030468
Rico-Pérez V, Bueno-López A. Effect of RhOx/CeO2 Calcination on Metal-Support Interaction and Catalytic Activity for N2O Decomposition. Applied Sciences. 2014; 4(3):468-481. https://0-doi-org.brum.beds.ac.uk/10.3390/app4030468
Chicago/Turabian StyleRico-Pérez, Verónica, and Agustin Bueno-López. 2014. "Effect of RhOx/CeO2 Calcination on Metal-Support Interaction and Catalytic Activity for N2O Decomposition" Applied Sciences 4, no. 3: 468-481. https://0-doi-org.brum.beds.ac.uk/10.3390/app4030468