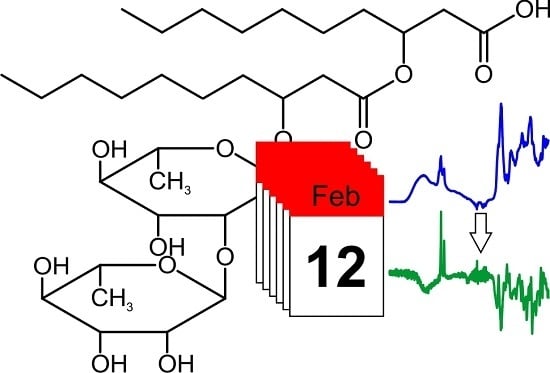
Infrared Spectroscopy for Studying Structure and Aging Effects in Rhamnolipid Biosurfactants
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process. Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Silva, S.N.R.L.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf. B 2010, 79, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Behrens, B.; Helmer, P.O.; Tiso, T.; Blank, L.M.; Hayen, H. Rhamnolipid biosurfactant analysis using on-line turbulent flow chromatography-liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1465, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Behrens, B.; Engelen, J.; Tiso, T.; Blank, L.M.; Hayen, H. Characterization of rhamnolipids by liquid chromatography/mass spectrometry after solid-phase extraction. Anal. Bioanal. Chem. 2016, 408, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Noack, K.; Eskofier, B.; Kiefer, J.; Dilk, C.; Bilow, G.; Schirmer, M.; Buchholz, R.; Leipertz, A. Combined shifted-excitation Raman difference spectroscopy and support vector regression for monitoring the algal production of complex polysaccharides. Analyst 2013, 138, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Posch, A.E.; Herwig, C.; Lendl, B. Comparison of Fiber Optic and Conduit Attenuated Total Reflection (ATR) Fourier Transform Infrared (FT-IR) Setup for In-Line Fermentation Monitoring. Appl. Spectrosc. 2016, 70, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fu, H.Y.; Zhou, W.C.; Hu, W.S. Advances in process monitoring tools for cell culture bioprocesses. Eng. Life Sci. 2015, 15, 459–468. [Google Scholar] [CrossRef]
- Griffiths, P.R.; De Haseth, J.A. Fourier Transform Infrared Spectrometry, 2nd ed.; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Kiefer, J.; Frank, K.; Schuchmann, H.P. Attenuated total reflection infrared (ATR-IR) spectroscopy of a water-in-oil emulsion. Appl. Spectrosc. 2011, 65, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Averett, L.A.; Griffiths, P.R. Effective path length in attenuated total reflection spectroscopy. Anal. Chem. 2008, 80, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bi, H.Y.; Jin, Y.S. Facile preparation of rhamnolipid-layered double hydroxide nanocomposite for simultaneous adsorption of p-cresol and copper ions from water. Chem. Eng. J. 2017, 308, 78–88. [Google Scholar] [CrossRef]
- Lahkar, J.; Borah, S.N.; Deka, S.; Ahmed, G. Biosurfactant of Pseudomonas aeruginosa JS29 against Alternaria solani: The causal organism of early blight of tomato. Biocontrol 2015, 60, 401–411. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, J.; Shi, R.J.; Han, S.Q.; Ma, F.; Zhang, Y. Production of biosurfactant by a Pseudomonas aeruginosa isolate and its applicability to in situ microbial enhanced oil recovery under anoxic conditions. RSC Adv. 2015, 5, 36044–36050. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Mohamed, M.S.; Samak, N. Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Braz. J. Chem. Eng. 2014, 31, 867–880. [Google Scholar] [CrossRef]
- Singh, A.K.; Cameotra, S.S. Rhamnolipids Production by Multi-metal-Resistant and Plant-Growth-Promoting Rhizobacteria. Appl. Biochem. Biotechnol. 2013, 170, 1038–1056. [Google Scholar] [CrossRef] [PubMed]
- Raheb, J.; Hajipour, M.J. The Characterization of Biosurfactant Production Related to Energy Consumption of Biodesulfurization in Pseudomonas aeruginosa ATCC9027. Energy Sources A 2012, 34, 1391–1399. [Google Scholar] [CrossRef]
- Arutchelvi, J.; Doble, M. Characterization of glycolipid biosurfactant from Pseudomonas aeruginosa CPCL isolated from petroleum-contaminated soil. Lett. Appl. Microbiol. 2010, 51, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Radzuan, M.N.; Banat, I.M.; Winterburn, J. Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour. Technol. 2017, 225, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Wu, G.; Yang, S.Z.; Mu, B.Z. Structural characterization of rhamnolipid produced by Pseudonomas aeruginosa strain FIN2 isolated from oil reservoir water. World J. Microbiol. Biotechnol. 2014, 30, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Jayachandran, K. Analysis of Rhamnolipid Biosurfactants Produced Through Submerged Fermentation Using Orange Fruit Peelings as Sole Carbon Source. Appl. Biochem. Biotechnol. 2009, 158, 694–705. [Google Scholar] [CrossRef] [PubMed]



| R1 | R2 | R3 | R4 | R5 | Assignment |
|---|---|---|---|---|---|
| ν/cm−1 | |||||
| 3274 | 3274 | 3288 | 3288 | 3285 | OH str |
| 2954s | 2954s | 2954s | 2954s | 2954s | CH3 asym str |
| 2926 | 2926 | 2926 | 2927s | 2926 | CH2 asym str |
| - | - | - | 2916 | - | CH2 asym str |
| 2871s | 2871s | 2871s | 2871s | 2871s | CH3 sym str |
| 2855 | 2855 | 2855 | 2850 | 2855 | CH2 sym str |
| 1740s | 1740s | 1740s | 1740s | 1740s | C=O str ester |
| 1707 | 1707 | 1707 | 1707 | 1707 | C=O str acid |
| 1669 | 1669 | 1647 | 1647 | 1647 | OH bend (residual water) |
| 1592 | 1592 | 1590s | 1591s | 1591s | COO asym str |
| 1527 | 1527 | 1527 | 1527 | 1527 | Residual CHCl3 |
| 1484 | 1484 | 1484s | - | - | Residual HCCl3 |
| 1442 | 1442 | 1442 | 1442 | 1442 | CH2 sciss |
| 1380 | 1380 | 1380s | 1380s | 1380s | COO sym str |
| 1292 | 1292 | 1297s | 1301s | 1301 | CH2 wag, COH bend |
| 1276s | 1277 | 1277 | 1274 | 1273 | CH2 wag, COH bend |
| 1241 | 1242 | 1242s | 1242s | 1242s | |
| 1214 | 1214 | 1207 | 1206 | 1206 | Residual CHCl3 |
| 1155 | 1155 | 1155 | 1156 | 1156 | CH rock |
| 1120 | 1120 | 1121 | 1121 | 1121 | CO str, CH rock |
| 1032 | 1032 | 1031 | 1031 | 1031 | CO str |
| 981 | 981 | 983 | 983 | 983 | |
| 964 | 964 | 965 | 965 | 966 | |
| 954s | 954s | - | - | - | |
| 918 | 918 | 917 | 917 | 918 | |
| 849 | 849 | 849 | 850s | 850s | |
| 818 | 818 | 818s | 818s | 818s | |
| 809 | 809 | 809 | 809 | 809 | |
| 752 | 753 | 755 | 756 | 756 | residual CHCl3 |
| - | 735s | - | - | - | CH rock |
| 724s | 723s | 724 | 724 | 724 | CH rock |
| 700 | 701 | 701 | 701 | 701 | CH rock |
| Rhamnolipid | m/z Value | Relative Intensity | Molecule Structure |
|---|---|---|---|
| Mono-rhamnolipid | 503 | 15 | Rha-C10-C10 |
| Di-rhamnolipid | 479 | 33 | Rha-Rha-C10 |
| 649 | 100 | Rha-Rha-C10-C10 | |
| 677 | 14 | Rha-Rha-C12-C10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, J.; Radzuan, M.N.; Winterburn, J. Infrared Spectroscopy for Studying Structure and Aging Effects in Rhamnolipid Biosurfactants. Appl. Sci. 2017, 7, 533. https://0-doi-org.brum.beds.ac.uk/10.3390/app7050533
Kiefer J, Radzuan MN, Winterburn J. Infrared Spectroscopy for Studying Structure and Aging Effects in Rhamnolipid Biosurfactants. Applied Sciences. 2017; 7(5):533. https://0-doi-org.brum.beds.ac.uk/10.3390/app7050533
Chicago/Turabian StyleKiefer, Johannes, Mohd Nazren Radzuan, and James Winterburn. 2017. "Infrared Spectroscopy for Studying Structure and Aging Effects in Rhamnolipid Biosurfactants" Applied Sciences 7, no. 5: 533. https://0-doi-org.brum.beds.ac.uk/10.3390/app7050533