Can OCT Angiography Be Made a Quantitative Blood Measurement Tool?
Abstract
:Featured Application
Abstract
1. Introduction
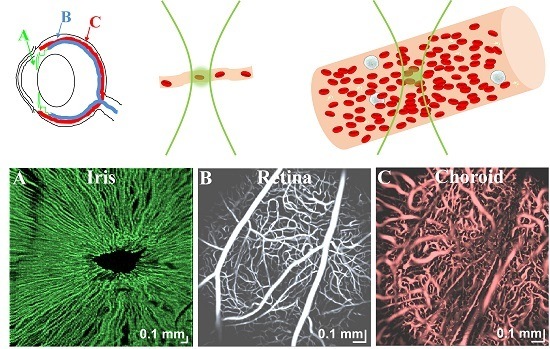
2. OCTA Fundamentals
2.1. Hemodynamic Parameters
2.2. Light Scattering from Red Blood Cells
3. OCTA Signal
4. OCTA Algorithms
4.1. Intensity- or Amplitude-Based OCTA Algorithms
4.2. Phase-Based OCTA Algorithms
4.3. Complex Signal-Based OCTA Algorithms
4.4. Classification of Present OCTA Algorithms
5. OCTA Scanning Protocols
6. Empirical Validation of OCTA
7. OCTA Measurements of Hemodynamics
7.1. Flow Quantification
7.2. Hematocrit Quantification
8. Can OCTA Be Made a Quantitative Tool?
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fedosov, D.A.; Noguchi, H.; Gompper, G. Multiscale modeling of blood flow: From single cells to blood rheology. Biomech. Model. Mechanobiol. 2014, 13, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W. Blood flow in the microcirculation. Annu. Rev. Fluid Mech. 2017, 49, 443–461. [Google Scholar] [CrossRef]
- Fung, Y.-C. Biomechanics: Motion, Flow, Stress, and Growth; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Choi, W.; Mohler, K.J.; Potsaid, B.; Lu, C.D.; Liu, J.J.; Jayaraman, V.; Cable, A.E.; Duker, J.S.; Huber, R.; Fujimoto, J.G. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed oct angiography. PLoS ONE 2013, 8, e81499. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F.; et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 2014, 121, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am. J. Ophthalmol. 2015, 160, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wei, E.; Wang, X.; Zhang, X.; Morrison, J.C.; Parikh, M.; Lombardi, L.H.; Gattey, D.M.; Armour, R.L.; Edmunds, B.; et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014, 121, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Fingler, J.; Zawadzki, R.J.; Park, S.S.; Morse, L.S.; Schwartz, D.M.; Fraser, S.E.; Werner, J.S. Optical imaging of the chorioretinal vasculature in the living human eye. Proc. Natl. Acad. Sci. USA 2013, 110, 14354–14359. [Google Scholar] [CrossRef] [PubMed]
- Talisa, E.; Bonini Filho, M.A.; Chin, A.T.; Adhi, M.; Ferrara, D.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Duker, J.S.; Waheed, N.K. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology 2015, 122, 1228–1238. [Google Scholar]
- Tsai, T.H.; Ahsen, O.O.; Lee, H.C.; Liang, K.; Figueiredo, M.; Tao, Y.K.; Giacomelli, M.G.; Potsaid, B.M.; Jayaraman, V.; Huang, Q.; et al. Endoscopic optical coherence angiography enables 3-dimensional visualization of subsurface microvasculature. Gastroenterology 2014, 147, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Ahsen, O.O.; Liang, K.; Wang, Z.; Cleveland, C.; Booth, L.; Potsaid, B.; Jayaraman, V.; Cable, A.E.; Mashimo, H.; et al. Circumferential optical coherence tomography angiography imaging of the swine esophagus using a micromotor balloon catheter. Biomed. Opt. Express 2016, 7, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Zhi, Z.; Wang, R.K. Three-dimensional optical imaging of microvascular networks within intact lymph node in vivo. J. Biomed. Opt. 2010, 15, 050501–050503. [Google Scholar] [CrossRef] [PubMed]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.J.; Jiang, J.Y.; Yaseen, M.A.; Radhakrishnan, H.; Wu, W.; Barry, S.; Cable, A.E.; Boas, D.A. Rapid volumetric angiography of cortical microvasculature with optical coherence tomography. Opt. Lett. 2010, 35, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.J.; Atochin, D.N.; Radhakrishnan, H.; Jiang, J.Y.; Ruvinskaya, S.; Wu, W.; Barry, S.; Cable, A.E.; Ayata, C.; Huang, P.L.; et al. Optical coherence tomography for the quantitative study of cerebrovascular physiology. J. Cereb. Blood Flow Metab. 2011, 31, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.J.; Radhakrishnan, H.; Lo, E.H.; Mandeville, E.T.; Jiang, J.Y.; Barry, S.; Cable, A.E. Oct methods for capillary velocimetry. Biomed. Opt. Express 2012, 3, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R. Autocorrelation optical coherence tomography for mapping transverse particle-flow velocity. Opt. Lett. 2010, 35, 3538–3540. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Moult, E.M.; Waheed, N.K.; Adhi, M.; Lee, B.; Lu, C.D.; de Carlo, T.E.; Jayaraman, V.; Rosenfeld, P.J.; Duker, J.S.; et al. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology 2015, 122, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.D.; Kirkpatrick, S.J. Can laser speckle flowmetry be made a quantitative tool? J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2008, 25, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Kleinfeld, D.; Mitra, P.P.; Helmchen, F.; Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl. Acad. Sci. USA 1998, 95, 15741–15746. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, W.S.; Chae, S.S.; Lacorre, D.A.; Tyrrell, J.A.; Mitre, M.; Gillissen, M.A.; Fukumura, D.; Jain, R.K.; Munn, L.L. Simultaneous measurement of rbc velocity, flux, hematocrit and shear rate in vascular networks. Nat. Method. 2010, 7, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Santisakultarm, T.P.; Cornelius, N.R.; Nishimura, N.; Schafer, A.I.; Silver, R.T.; Doerschuk, P.C.; Olbricht, W.L.; Schaffer, C.B. In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in mice. Am. J. Physiol. Heart Circ. Phys. 2012, 302, H1367–H1377. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, C.; Duling, B.R. Microvessel hematocrit: Measurement and implications for capillary oxygen transport. Am. J. Physiol. Heart Circ. Physiol. 1987, 252, H494–H503. [Google Scholar]
- Faber, D.J.; Aalders, M.C.; Mik, E.G.; Hooper, B.A.; van Gemert, M.J.; van Leeuwen, T.G. Oxygen saturation-dependent absorption and scattering of blood. Phys. Rev. Lett. 2004, 93, 028102. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.; Müller, G.; Helfmann, J.; Friebel, M. Optical properties of platelets and blood plasma and their influence on the optical behavior of whole blood in the visible to near infrared wavelength range. J. Biomed. Opt. 2007, 12, 014024–014029. [Google Scholar] [CrossRef] [PubMed]
- Sydoruk, O.; Zhernovaya, O.; Tuchin, V.; Douplik, A. Refractive index of solutions of human hemoglobin from the near-infrared to the ultraviolet range: Kramers-kronig analysis. J. Biomed. Opt. 2012, 17, 115002. [Google Scholar] [CrossRef] [PubMed]
- Bosschaart, N.; Edelman, G.J.; Aalders, M.C.; van Leeuwen, T.G.; Faber, D.J. A literature review and novel theoretical approach on the optical properties of whole blood. Lasers Med. Sci. 2014, 29, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavsky, A.N.; Yaroslavsky, I.V.; Goldbach, T.; Schwarzmaier, H.-J. Optical properties of blood in the near-infrared spectral range. Proceedings of Photonics West 1996, San Jose, CA, USA, 17 May 1996; pp. 314–324. [Google Scholar]
- Simon, J.-C. Dependent scattering and radiative transfer in dense inhomogeneous media. Phys. A Stat. Mech. Appl. 1997, 241, 77–81. [Google Scholar] [CrossRef]
- Roggan, A.; Friebel, M.; Dörschel, K.; Hahn, A.; Muller, G. Optical properties of circulating human blood in the wavelength range 400–2500 nm. J. Biomed. Opt. 1999, 4, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Friebel, M.; Roggan, A.; Müller, G.; Meinke, M. Determination of optical properties of human blood in the spectral range 250 to 1100 nm using monte carlo simulations with hematocrit-dependent effective scattering phase functions. J. Biomed. Opt. 2006, 11, 034021. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.; Müller, G.; Helfmann, J.; Friebel, M. Empirical model functions to calculate hematocrit-dependent optical properties of human blood. Appl. Opt. 2007, 46, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Henyey, L.G.; Greenstein, J.L. Diffuse radiation in the galaxy. Astrophys. J. 1941, 93, 70–83. [Google Scholar] [CrossRef]
- Hammer, M.; Yaroslavsky, A.N.; Schweitzer, D. A scattering phase function for blood with physiological haematocrit. Phys. Med. Biol. 2001, 46, N65. [Google Scholar] [CrossRef] [PubMed]
- Cimalla, P.; Walther, J.; Mittasch, M.; Koch, E. Shear flow-induced optical inhomogeneity of blood assessed in vivo and in vitro by spectral domain optical coherence tomography in the 1.3 μm wavelength range. J. Biomed. Opt. 2011, 16, 116020. [Google Scholar] [CrossRef] [PubMed]
- Friebel, M.; Helfmann, J.; Müller, G.; Meinke, M. Influence of shear rate on the optical properties of human blood in the spectral range 250 to 1100 nm. J. Biomed. Opt. 2007, 12, 054005–054008. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.-K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010, 31, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Bigio, I.J.; Fantini, S. Quantitative Biomedical Optics: Theory, Methods, and Applications; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Chen, C.-L.; Wang, R.K. Optical coherence tomography based angiography [invited]. Biomed. Opt. Express 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (octa). Int. J. Retina Vitreous 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Siegert, A. On the Fluctuations in Signals Returned by Many Independently Moving Scatterers; Massachusetts Institute of Technology: Cambridge, MA, USA, 1943. [Google Scholar]
- Srinivasan, V.J.; Chan, A.C.; Lam, E.Y. Doppler OCT and OCT Angiography for In Vivo Imaging of Vascular Physiology; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Leitgeb, R.A.; Werkmeister, R.M.; Blatter, C.; Schmetterer, L. Doppler optical coherence tomography. Prog. Retinal Eye Res. 2014, 41, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Xiang, S.; Yung, K.M. Speckle in optical coherence tomography. J. Biomed. Opt. 1999, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Stromski, S. Flow measurement without phase information in optical coherence tomography images. Opt. Express 2005, 13, 5234–5239. [Google Scholar] [CrossRef] [PubMed]
- Mariampillai, A.; Standish, B.A.; Moriyama, E.H.; Khurana, M.; Munce, N.R.; Leung, M.K.; Jiang, J.; Cable, A.; Wilson, B.C.; Vitkin, I.A. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt. Lett. 2008, 33, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Mariampillai, A.; Leung, M.K.; Jarvi, M.; Standish, B.A.; Lee, K.; Wilson, B.C.; Vitkin, A.; Yang, V.X. Optimized speckle variance oct imaging of microvasculature. Opt. Lett. 2010, 35, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Enfield, J.; Jonathan, E.; Leahy, M. In vivo imaging of the microcirculation of the volar forearm using correlation mapping optical coherence tomography (cmoct). Biomed. Opt. Express 2011, 2, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.A.; Schmetterer, L.; Drexler, W.; Fercher, A.; Zawadzki, R.; Bajraszewski, T. Real-time assessment of retinal blood flow with ultrafast acquisition by color doppler fourier domain optical coherence tomography. Opt. Express 2003, 11, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.A.; Schmetterer, L.; Hitzenberger, C.K.; Fercher, A.F.; Berisha, F.; Wojtkowski, M.; Bajraszewski, T. Real-time measurement of in vitro flow by fourier-domain color doppler optical coherence tomography. Opt. Lett. 2004, 29, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Z.; Saxer, C.; Shen, Q.; Xiang, S.; de Boer, J.F.; Nelson, J.S. Doppler standard deviation imaging for clinical monitoring of in vivo human skin blood flow. Opt. Lett. 2000, 25, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Makita, S.; Hong, Y.; Yamanari, M.; Yatagai, T.; Yasuno, Y. Optical coherence angiography. Opt. Express 2006, 14, 7821–7840. [Google Scholar] [CrossRef] [PubMed]
- Park, B.H.; Pierce, M.C.; Cense, B.; Yun, S.-H.; Mujat, M.; Tearney, G.J.; Bouma, B.E.; de Boer, J.F. Real-time fiber-based multi-functional spectral-domain optical coherence tomography at 1.3 µm. Opt. Express 2005, 13, 3931–3944. [Google Scholar] [CrossRef] [PubMed]
- Fingler, J.; Schwartz, D.; Yang, C.; Fraser, S.E. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt. Express 2007, 15, 12636–12653. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Fingler, J.; Werner, J.S.; Schwartz, D.M.; Fraser, S.E.; Zawadzki, R.J. In vivo volumetric imaging of human retinal circulation with phase-variance optical coherence tomography. Biomed. Opt. Express 2011, 2, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Srinivasan, V.; Radhakrishnan, H.; Boas, D.A. Motion correction for phase-resolved dynamic optical coherence tomography imaging of rodent cerebral cortex. Opt. Express 2011, 19, 21258–21270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Chen, C.-L.; Wang, R.K. Methods and algorithms for optical coherence tomography-based angiography: A review and comparison. J. Biomed. Opt. 2015, 20, 100901. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Jacques, S.L.; Ma, Z.; Hurst, S.; Hanson, S.R.; Gruber, A. Three dimensional optical angiography. Opt. Express 2007, 15, 4083–4097. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Qin, J.; Wang, R.K. Ultrahigh sensitive optical microangiography for in vivo imaging of microcirculations within human skin tissue beds. Opt. Express 2010, 18, 8220–8228. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.S.; Chico-Calero, I.; Vakoc, B.J. Complex differential variance algorithm for optical coherence tomography angiography. Biomed. Opt. Express 2014, 5, 3822–3832. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.W. Statistical Optics; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Wei, W.; Xu, J.; Baran, U.; Song, S.; Qin, W.; Qi, X.; Wang, R.K. Intervolume analysis to achieve four-dimensional optical microangiography for observation of dynamic blood flow. J. Biomed. Opt. 2016, 21, 36005. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Wieser, W.; Reznicek, L.; Neubauer, A.; Kampik, A.; Huber, R. Multi-mhz retinal oct. Biomed. Opt. Express 2013, 4, 1890–1908. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Qin, W.; Chen, C.L.; Wang, J.; Zhang, Q.; Yang, X.; Gao, B.Z.; Wang, R.K. Characterizing relationship between optical microangiography signals and capillary flow using microfluidic channels. Biomed. Opt. Express 2016, 7, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, M.; Myllylä, R. Effect of glucose on photoacoustic signals at the wavelengths of 1064 and 532 nm in pig blood and intralipid. J. Phys. D Appl. Phys. 2005, 38, 2654. [Google Scholar] [CrossRef]
- Van Staveren, H.J.; Moes, C.J.; van Marie, J.; Prahl, S.A.; Van Gemert, M.J. Light scattering in lntralipid-10% in the wavelength range of 400–1100 nm. Appl. Opt. 1991, 30, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Su, J.P.; Chandwani, R.; Gao, S.S.; Pechauer, A.D.; Zhang, M.; Wang, J.; Jia, Y.; Huang, D.; Liu, G. Calibration of optical coherence tomography angiography with a microfluidic chip. J. Biomed. Opt. 2016, 21, 86015. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Baran, U.; Li, Y.; Qin, W.; Wang, W.; Zeng, H.; Wang, R.K. Does optical microangiography provide accurate imaging of capillary vessels?: Validation using multiphoton microscopy. J. Biomed. Opt. 2014, 19, 106011. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Du, C.; Park, K.; Volkow, N.D.; Pan, Y. Quantitative imaging of red blood cell velocity invivo using optical coherence doppler tomography. Appl. Phys. Lett. 2012, 100, 233702. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Du, C.; Yuan, Z.; Park, K.; Volkow, N.D.; Pan, Y. Cocaine-induced cortical microischemia in the rodent brain: Clinical implications. Mol. Psychiatry 2012, 17, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Merkle, C.W.; Lam, E.Y.; Srinivasan, V.J. Maximum Likelihood Estimation of Blood Velocity Using Doppler Optical Coherence Tomography; SPIE BiOS: Bellingham, WA, USA, 2014; p. 89349. [Google Scholar]
- Srinivasan, V.J.; Sakadžić, S.; Gorczynska, I.; Ruvinskaya, S.; Wu, W.; Fujimoto, J.G.; Boas, D.A. Quantitative cerebral blood flow with optical coherence tomography. Opt. Express 2010, 18, 2477–2494. [Google Scholar] [CrossRef] [PubMed]
- Ploner, S.B.; Moult, E.M.; Choi, W.; Waheed, N.K.; Lee, B.; Novais, E.A.; Cole, E.D.; Potsaid, B.; Husvogt, L.; Schottenhamml, J. Toward quantitative optical coherence tomography angiography: Visualizing blood flow speeds in ocular pathology using variable interscan time analysis. Retina 2016, 36, S118–S126. [Google Scholar] [CrossRef] [PubMed]
- Bonner, R.; Nossal, R. Model for laser doppler measurements of blood flow in tissue. Appl. Opt. 1981, 20, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.J.; van der Meer, F.J.; Aalders, M.C.; van Leeuwen, T.G. Hematocrit-dependence of the scattering coefficient of blood determined by optical coherence tomography. In Proceedings of the Saratov Fall Meeting 2005: Optical Technologies in Biophysics and Medicine VII, Saratov, Russia, 27–30 October 2005; SPIE: Bellingham, WA, USA, 2006; p. 61639. [Google Scholar]
- Faber, D.J.; van Leeuwen, T.G. Are quantitative attenuation measurements of blood by optical coherence tomography feasible? Opt Lett. 2009, 34, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.J.; Radhakrishnan, H. Optical coherence tomography angiography reveals laminar microvascular hemodynamics in the rat somatosensory cortex during activation. NeuroImage 2014, 102, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Tokayer, J.; Jia, Y.; Dhalla, A.-H.; Huang, D. Blood flow velocity quantification using split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Biomed. Opt. Express 2013, 4, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Li, Y.; Qin, W.; Wang, R.K. Cerebral capillary velocimetry based on temporal oct speckle contrast. Biomed. Opt. Express 2016, 7, 4859–4873. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, A.E.; Nam, A.S.; Chico-Calero, I.; Vakoc, B.J. Monte carlo modeling of angiographic optical coherence tomography. Biomed. Opt. Express 2014, 5, 4338–4349. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Chen, S.; Backman, V.; Zhang, H.F. In vivo functional microangiography by visible-light optical coherence tomography. Biomed. Opt. Express 2014, 5, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.P.; Merkle, C.W.; Leahy, C.; Radhakrishnan, H.; Srinivasan, V.J. Quantitative microvascular hemoglobin mapping using visible light spectroscopic optical coherence tomography. Biomed. Opt. Express 2015, 6, 1429–1450. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; You, J.; Volkow, N.D.; Park, K.; Du, C. Ultrasensitive detection of 3d cerebral microvascular network dynamics in vivo. Neuroimage 2014, 103, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Merkle, C.W.; Leahy, C.; Srinivasan, V.J. Dynamic contrast optical coherence tomography images transit time and quantifies microvascular plasma volume and flow in the retina and choriocapillaris. Biomed. Opt. Express 2016, 7, 4289–4312. [Google Scholar] [CrossRef] [PubMed]
- Merkle, C.W.; Srinivasan, V.J. Laminar microvascular transit time distribution in the mouse somatosensory cortex revealed by dynamic contrast optical coherence tomography. Neuroimage 2016, 125, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Assadi, H.; Demidov, V.; Karshafian, R.; Douplik, A.; Vitkin, I.A. Microvascular contrast enhancement in optical coherence tomography using microbubbles. J. Biomed. Opt. 2016, 21, 076014. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Hoying, J.B.; Sullivan, C.J. Use of microbubbles as an optical coherence tomography contrast agent. Acad. Radiol. 2002, 9, S52–S55. [Google Scholar] [CrossRef]










| Symbol | Meaning |
|---|---|
| Complex OCT signal/field | |
| Amplitude of the OCT signal | |
| Intensity of the OCT signal | |
| Phase of the OCT signal | |
| OCT field from one scatterer | |
| Speckle variance | |
| Correlation mapping OCT signal | |
| Phase variance | |
| Phase difference | |
| Complex field difference | |
| Complex differential variance | |
| Autocorrelation function | |
| Power spectral density |
| Category | Classification |
|---|---|
| OCT signal | Field vs. Intensity/Amplitude vs. Phase |
| Calculation | Variance/Difference vs. Correlation |
| Averaging method | Temporal vs. Spatial vs. Spectral |
| Normalization | Normalized vs. Non-normalized |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Merkle, C.W.; Bernucci, M.T.; Chong, S.P.; Srinivasan, V.J. Can OCT Angiography Be Made a Quantitative Blood Measurement Tool? Appl. Sci. 2017, 7, 687. https://0-doi-org.brum.beds.ac.uk/10.3390/app7070687
Zhu J, Merkle CW, Bernucci MT, Chong SP, Srinivasan VJ. Can OCT Angiography Be Made a Quantitative Blood Measurement Tool? Applied Sciences. 2017; 7(7):687. https://0-doi-org.brum.beds.ac.uk/10.3390/app7070687
Chicago/Turabian StyleZhu, Jun, Conrad W. Merkle, Marcel T. Bernucci, Shau Poh Chong, and Vivek J. Srinivasan. 2017. "Can OCT Angiography Be Made a Quantitative Blood Measurement Tool?" Applied Sciences 7, no. 7: 687. https://0-doi-org.brum.beds.ac.uk/10.3390/app7070687