Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as Function of Biofilm Age
Abstract
:Featured Application
Abstract
1. Introduction
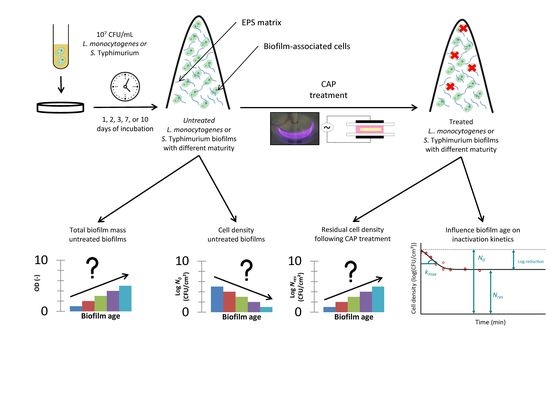
2. Materials and Methods
2.1. Experimental Design
2.2. Microorganisms, Pre-culture Conditions, and Inoculum Preparation
2.3. Development of Biofilms with Different Ages
2.4. CAP Equipment and Inactivation Procedure
2.5. Biofilm Quantification Via Viable Cell Counts
2.6. Biofilm Quantification Via Crystal Violet Staining
2.7. Statistical Analysis, Modelling, Parameter Estimation, and Estimation of Sub-Lethal Injury
3. Results and Discussion
3.1. Influence of the Biofilm Age on the Total Biofilm Mass and the Cell Density of the Untreated Biofilms
3.2. Influence of the Biofilm Age on the CAP Efficacy Following 10 min of Treatment
3.3. Influence of the Biofilm Age on the Inactivation Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Barry, D.M.; Kanematsu, H. Cooling Water. In Biofilm and Materials Science, 1st ed.; Kanematsu, H., Barry, D.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 79–84. ISBN 978-3-319-14564-8. [Google Scholar]
- Javaherdashti, R. Corrosion and Biofilm. In Biofilm and Materials Science, 1st ed.; Kanematsu, H., Barry, D.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 69–78. ISBN 978-3-319-14564-8. [Google Scholar]
- Kim, S.; Wei, C. Biofilms. In Decontamination of Fresh and Minimally Processed Produce, 1st ed.; Gómez-López, V.M., Ed.; John Wiley & Sons, Inc.: Ames, IA, USA, 2012; pp. 59–76. ISBN 978-0-8138-2384-3. [Google Scholar]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2017, 14, 3991. [Google Scholar] [CrossRef]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Antibiotic Tolerance and Resistance in Biofilms. In Biofilm Infections, 1st ed.; Bjarnsholt, T., Jensen, P.Ø., Moser, C., Høiby, N., Eds.; Springer: New York, NY, USA, 2001; pp. 215–229. ISBN 978-1-489-98228-5. [Google Scholar]
- Anwar, H.; Costerton, J.W. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1990, 34, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Fraud, S.; Maillard, J.Y.; Kaminski, M.A.; Hanlon, G.W. Activity of amine oxide against biofilms of Streptococcus mutans: A potential biocide for oral care formulations. J. Antimicrob. Chemother. 2005, 56, 672–677. [Google Scholar] [CrossRef]
- Stewart, S.P. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 1–30. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, S.; Zhang, C.; Zhao, X.; Huang, X.; Cai, Z. Effect of the Biofilm Age and Starvation on Acid Tolerance of Biofilm formed by Streptococcus mutans Isolated from Caries-Active and Caries-Free Adults. Int. J. Mol. Sci. 2017, 18, 713. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Han, L.; Cullen, P.J.; Bourke, P. Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli. Int. J. Food Microbiol. 2015, 210, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Govaert, M.; Smet, C.; Vergauwen, L.; Ećimović, E.; Walsh, J.L.; Baka, M.; Van Impe, J. Influence of plasma characteristics on the efficacy of Cold Atmospheric Plasma (CAP) for inactivation of Listeria monocytogenes and Salmonella Typhimurium biofilms. Innov. Food Sci. Emerg. Technol. 2018, 2018, 221–228. [Google Scholar]
- Fernández, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Food Res. Int. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Banu, M.S.; Sasikala, P.; Dhanapal, A.; Kavitha, V.; Yazhini, G.; Rajamani, L. Cold plasma as a novel food processing technology. IJETED 2012, 4, 803–818. [Google Scholar]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Bourke, P.; Cullen, P.J. Principles of Nonthermal Plasma Decontamination. In Cold Plasma in Food and Agriculture—Fundamentals and Applications, 1st ed.; Misra, N.N., Schlüter, O.K., Cullen, P.J., Eds.; Elsevier Inc.: London, UK, 2016; pp. 143–178. ISBN 978-0-12-801365-6. [Google Scholar]
- Wuytack, E.Y.; Phuong, L.D.; Aertsen, A.; Reyns, K.M.; Marquenie, D.; De Ketelaere, B.; Masschalck, B.; Van Opstal, I.; Diels, A.M.; Michiels, C.W. Comparison of sublethal injury induced in Salmonella enterica serovar Typhimurium by heat and by different nonthermal treatments. J. Food Prot. 2003, 66, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Noriega, E.; Velliou, E.; Van Derlinden, E.; Mertens, L.; Van Impe, J.F. Effect of cell immobilization on heat-induced sublethal injury of Escherichia coli, Salmonella Typhimurium and Listeria innocua. Food Microbiol. 2013, 36, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Devlieghere, F.; Geeraerd, A.; Uyttendaele, M. Thermal inactivation and sublethal injury kinetics of Salmonella enterica and Listeria monocytogenes in broth versus agar surface. Int. J. Food Microbiol. 2017, 243, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Govaert, M.; Smet, C.; Baka, M.; Janssens, T.; Van Impe, J. Influence of incubation conditions on the formation of model biofilms by Listeria monocytogenes and Salmonella Typhimurium on abiotic surfaces. J. Appl. Microbiol. 2018, 125, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of bactericidal power of the blood. J. Hyg. (Lond.) 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Busch, S.V.; Donnelly, C.W. Development of a repair-enrichment broth for resuscitation of heat-injured Listeria monocytogenes and Listeria innocua. Appl. Environ. Microbiol. 1992, 58, 14–20. [Google Scholar] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.C. Adhesion and growth of bacteria at surfaces in oligotrophic habitats. Can. J. Microbiol. 1988, 34, 503–506. [Google Scholar] [CrossRef]
- Lappin-Scott, H.M.; Bass, C. Biofilm formation: Attachment, growth, and detachment of microbes from surfaces. Am. J. Infect. Control 2001, 29, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Wilson, S.; Hall-Stoodley, L.; Boyle, J.D.; Lappin-Scott, H.M.; Costerton, J.W. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl. Environ. Microbiol. 2001, 67, 5608–5613. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005, 57, 1210–1223. [Google Scholar] [CrossRef]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog 2009, 5, e1000354. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT-Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.G. Biofilm dispersion. In Biofilm Highlights, 1st ed.; Flemming, H.-C., Wingender, J., Szewzyk, U., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 1–28. [Google Scholar]
- Anwar, H.; Strap, J.L.; Costerton, J.W. Establishment of Aging Biofilms: Possible Mechanism of Bacterial Resistance to Antimicrobial Therapy. Antimicrob. Agents Chemother. 1992, 36, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms on Central Venous Catheters: Is Eradication Possible. In Current Topics in Microbiology and Immunology, 1st ed.; Romeo, T., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2008; Volume 322, pp. 133–162. ISBN 978-3-540-75417-6. [Google Scholar]
- Wood, S.R.; Kirkham, J.; Shore, R.C.; Brookes, S.J.; Robinson, C. Changes in the structure and density of oral plaque biofilms with increasing plaque age. FEMS Microbiol. Ecol. 2002, 39, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smet, C.; Noriega, E.; Rosier, F.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Influence of food intrinsic factors on the inactivation efficacy of cold atmospheric plasma: Impact of osmotic stress, suboptimal pH and food structure. Innov. Food Sci. Emerg. Technol. 2016, 38, 393–406. [Google Scholar] [CrossRef]





| Model Parameters | L. monocytogenes | S. Typhimurium | ||
|---|---|---|---|---|
| One-Day Old | 7 Days Old | One-Day Old | 7 Days Old | |
| 1 Log10 N0 general medium (log(CFU/cm2)) | B 7.145 ± 0.071 | A 6.151 ± 0.220 | A 6.431 ± 0.068 | A 5.857 ± 0.229 |
| 1 Log10 N0 selective medium (log(CFU/cm2)) | B 7.154 ± 0.071 | A 6.218 ± 0.237 | A 6.140 ± 0.089 | A 5.397 ± 0.249 |
| 1kmax general medium (1/min) | B 1.265 ± 0.142 | A 0.253 ± 0.057 | A 1.308 ± 0.154 | A 2.730 ± 0.785 |
| 1kmax selective medium (1/min) | B 1.735 ± 0.172 | A 0.278 ± 0.078 | A 1.534 ± 0.198 | A 2.598 ± 0.687 |
| 1 Log10 Nres general medium (log(CFU/cm2)) | A 3.504 ± 0.133 | A 2.681 ± 1.710 | A 2.972 ± 0.125 | A 3.474 ± 0.158 |
| 1 Log10 Nres selective medium (log(CFU/cm2)) | A 2.935 ± 0.132 | A 3.501 ± 0.557 | A 2.134 ± 0.161 | A 2.680 ± 0.174 |
| 1 Log-reduction general medium (log(CFU/cm2)) | A 3.641 ± 0.150 | A 3.470 ± 1.724 | B 3.460 ± 0.143 | A 2.383 ± 0.278 |
| 1 Log-reduction selective medium (log(CFU/cm2)) | B 4.219 ± 0.149 | A 2.717 ± 0.606 | B 4.006 ± 0.184 | A 2.716 ± 0.304 |
| RMSE general medium (/) | 0.4354 | 0.7330 | 0.4198 | 0.5714 |
| RMSE selective medium (/) | 0.4329 | 0.7620 | 0.5468 | 0.6263 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govaert, M.; Smet, C.; Baka, M.; Ećimović, B.; Walsh, J.L.; Van Impe, J. Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as Function of Biofilm Age. Appl. Sci. 2018, 8, 2702. https://0-doi-org.brum.beds.ac.uk/10.3390/app8122702
Govaert M, Smet C, Baka M, Ećimović B, Walsh JL, Van Impe J. Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as Function of Biofilm Age. Applied Sciences. 2018; 8(12):2702. https://0-doi-org.brum.beds.ac.uk/10.3390/app8122702
Chicago/Turabian StyleGovaert, Marlies, Cindy Smet, Maria Baka, Branimir Ećimović, James L. Walsh, and Jan Van Impe. 2018. "Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as Function of Biofilm Age" Applied Sciences 8, no. 12: 2702. https://0-doi-org.brum.beds.ac.uk/10.3390/app8122702