Blue Electrofluorescence Properties of Furan–Silole Ladder Pi-Conjugated Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Device Fabrication and Characterization
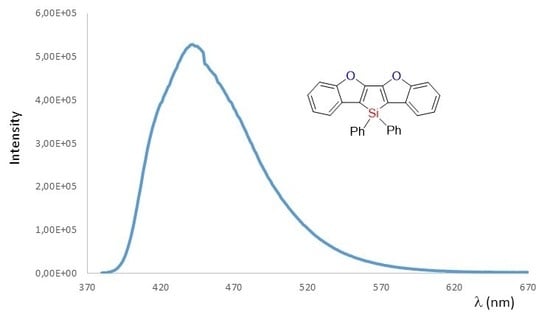
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Roncali, J. Synthetic principles for bandgap control in linear π-conjugated systems. Chem. Rev. 1997, 97, 173–206. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.-Q.; Bauerle, P. Functional oligothiophenes: Molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef] [PubMed]
- Hissler, M.; Dyer, P.W.; Réau, R. Linear organic π-conjugated systems featuring the heavy group 14 and 15 elements. Coord. Chem. Rev. 2003, 244, 1–44. [Google Scholar] [CrossRef]
- Entwistle, C.D.; Marder, T.B. Boron chemistry lights the way: Optical properties of molecular and polymeric systems. Angew. Chem. Int. Ed. 2002, 41, 2927–2931. [Google Scholar] [CrossRef]
- Dou, C.; Saito, S.; Matsuo, K.; Hisaki, I.; Yamaguchi, S. A Boron–Containing PAH as a Substructure of Boron-Doped Graphene. Angew. Chem. Int. Ed. 2012, 51, 12206–12210. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.F.; Kanazawa, K.K.; Gardini, G.P. Electrochemical polymerization of pyrrole. J. Chem. Soc. Chem. Commun. 1979, 14, 635–636. [Google Scholar] [CrossRef]
- Sun, M.; Wang, L.; Yang, W. Pyrrole-based narrow-band-gap copolymers for red light-emitting diodes and bulk heterojunction photovoltaic cells. J. Appl. Polym. Sci. 2010, 118, 1462–1468. [Google Scholar] [CrossRef]
- Gidron, O.; Diskin-Posner, Y.; Bendikov, M. α-Oligofurans. J. Am. Chem. Soc. 2010, 132, 2148–2150. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Beaujuge, P.M.; Holcombe, T.W.; Lee, O.P.; Fréchet, J.M.J. Incorporation of furan into low band-gap polymers for efficient solar cells. J. Am. Chem. Soc. 2010, 132, 15547–15549. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Barlow, S.; Marder, S.R. Substituent effects on the electronic structure of siloles. Chem. Commun. 2009, 15, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, T.; Réau, R. Organophosphorus π-conjugated materials. Chem. Rev. 2006, 106, 4681–4727. [Google Scholar] [CrossRef] [PubMed]
- Bouit, P.-A.; Escande, A.; Szűcs, R.; Szieberth, D.; Lescop, C.; Nyulászi, L.; Hissler, M.; Réau, R. Dibenzophosphapentaphenes: Exploiting P chemistry for gap fine-tuning and coordination-driven assembly of planar polycyclic aromatic hydrocarbons. J. Am. Chem. Soc. 2012, 134, 6524–6527. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Delaunay, W.; Yu, L.; Joly, D.; Wang, Z.; Li, J.; Wang, Z.; Lescop, C.; Tondelier, D.; Geffroy, B.; et al. 2, 2′-Biphospholes: Building Blocks for Tuning the HOMO–LUMO Gap of π-Systems Using Covalent Bonding and Metal Coordination. Angew. Chem. Int. Ed. 2012, 51, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pascal, S.; Wang, Z.; Bouit, P.-A.; Wang, Z.; Zhang, Y.; Tondelier, D.; Geffroy, B.; Réau, R.; Mathey, F.; et al. 1, 2-Dihydrophosphete: A Platform for the Molecular Engineering of Electroluminescent Phosphorus Materials for Light-Emitting Devices. Chem. Eur. J. 2014, 20, 9784–9793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Kan, W.H.; Henderson, M.A.; Bomben, P.G.; Berlinguette, C.P.; Thangadurai, V.; Baumgartner, T. External-stimuli responsive photophysics and liquid crystal properties of self-assembled “phosphole-lipids”. J. Am. Chem. Soc. 2011, 133, 17014–17026. [Google Scholar] [CrossRef] [PubMed]
- Matano, Y.; Saito, A.; Fukushima, T.; Tokudome, Y.; Suzuki, F.; Sakamaki, D.; Kaji, H.; Ito, A.; Tanaka, K.; Imahori, H. Fusion of Phosphole and 1, 1′-Biacenaphthene: Phosphorus (V)-Containing Extended π-Systems with High Electron Affinity and Electron Mobility. Angew. Chem. Int. Ed. 2011, 50, 8016–8020. [Google Scholar] [CrossRef] [PubMed]
- Joly, D.; Bouit, P.-A.; Hissler, M. Organophosphorus derivatives for electronic devices. J. Mater. Chem. C 2016, 4, 3686–3698. [Google Scholar] [CrossRef] [Green Version]
- Duffy, M.P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. π-Conjugated phospholes and their incorporation into devices: Components with a great deal of potential. Chem. Soc. Rev. 2016, 45, 5296–5310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szűcs, R.; Bouit, P.-A.; Nyulászi, L.; Hissler, M. P-containing Polycyclic Aromatic Hydrocarbons. Chem. Phys. Chem. 2017, 18, 2618–2630. [Google Scholar] [CrossRef] [PubMed]
- Tamao, K.; Uchida, M.; Izumizawa, T.; Furukawa, K.; Yamaguchi, S. Silole derivatives as efficient electron transporting materials. J. Am. Chem. Soc. 1996, 118, 11974–11975. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lam, W.Y.; Luo, J.D.; Ho, Y.L.; Tang, B.Z.; Zhu, D.B.; Wong, M.; Kwok, H.S. Highly efficient organic light-emitting diodes with a silole-based compound. Appl. Phys. Lett. 2002, 81, 574–576. [Google Scholar] [CrossRef]
- Geramita, K.; McBee, J.; Shen, Y.; Radu, N.; Tilley, T.D. Synthesis and characterization of perfluoroaryl-substituted siloles and thiophenes: A series of electron-deficient blue light emitting materials. Chem. Mater. 2006, 18, 3261–3269. [Google Scholar] [CrossRef]
- Lee, J.H.; Yuan, Y.Y.; Kang, Y.J.; Jia, W.L.; Lu, Z.H.; Wang, S.N. 2, 5-Functionalized Spiro-Bisiloles as Highly Efficient Yellow-Light Emitters in Electroluminescent Devices. Adv. Funct. Mater. 2006, 16, 681–686. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Xu, C.; Tamao, K. Bis-silicon-bridged stilbene homologues synthesized by new intramolecular reductive double cyclization. J. Am. Chem. Soc. 2003, 125, 13662–13663. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wakamiya, A.; Yamaguchi, S. Ladder Oligo(p-phenylenevinylene)s with Silicon and Carbon Bridges. J. Am. Chem. Soc. 2005, 127, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Mouri, K.; Wakamiya, A.; Yamada, H.; Kajiwara, T.; Yamaguchi, S. Ladder distyrylbenzenes with silicon and chalcogen bridges: Synthesis, structures, and properties. Org. Lett. 2006, 9, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Yamaguchi, S. Ladder π-Conjugated Materials Containing Main-Group Elements. Chem. Asian J. 2009, 4, 1386–1400. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J.; Lee, K.-H.; Kimura, K.; Kunai, A. Synthesis of siloles condensed with benzothiophene and indole rings. Organometallics 2004, 23, 5622–5625. [Google Scholar] [CrossRef]
- Wan, J.-H.; Fang, W.-F.; Li, Z.-F.; Xiao, X.-Q.; Xu, Z.; Deng, Y.; Zhang, L.-H.; Jiang, J.-X.; Qiu, H.-Y.; Wu, L.-B.; et al. Novel Ladder π-Conjugated Materials—Sila-Pentathienoacenes: Synthesis, Structure, and Electronic Properties. Chem. Asian J. 2010, 5, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Ureshino, T.; Yoshida, T.; Kuninobu, Y.; Takai, K. Rhodium-catalyzed synthesis of silafluorene derivatives via cleavage of silicon−hydrogen and carbon−hydrogen bonds. J. Am. Chem. Soc. 2010, 132, 14324–14326. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Wang, Z.H.; Gan, Z.J.; Xi, Q.Z.; Duan, Z.; Mathey, F. Versatile Synthesis of Phospholides from Open-Chain Precursors. Application to Annelated Pyrrole–and Silole–Phosphole Rings. Org. Lett. 2015, 17, 1732–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, S.; Li, J.; He, G.F.; Duan, Z.; Mathey, F. Phosphorus and silicon-bridged stilbenes: Synthesis and optoelectronic properties. Dalton Trans. 2016, 45, 18308–18312. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Nakano, K.; Harada, T.; Kuroda, R.; Naito, M.; Nobusawa, K.; Nozaki, K. Facile synthetic route to highly luminescent sil [7] helicene. Org. Lett. 2013, 15, 2104–2107. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Uchiyama, T.; Yoshinami, Y.; Takayasu, S.; Tsuchikama, K.; Endo, K. Highly enantioselective synthesis of silahelicenes using Ir-catalyzed [2+2+2] cycloaddition. Chem. Commun. 2012, 48, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Development of a Sila-Friedel− Crafts Reaction and Its Application to the Synthesis of Dibenzosilole Derivatives. J. Am. Chem. Soc. 2009, 131, 14192–14193. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Delaunay, W.; Li, J.; Wang, Z.; Bouit, P.-A.; Tondelier, D.; Geffroy, B.; Mathey, F.; Duan, Z.; Réau, R.; et al. Benzofuran-fused phosphole: Synthesis, electronic, and electroluminescence properties. Org. Lett. 2013, 15, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, W.; Ma, W.; Zang, Z.; Zhang, H.; Liu, X.; Zou, S.; Zhang, H.; Liu, W.; Gao, J. Easily-soluble heteroacene bis (benzothieno) silole derivatives for sensing of nitro explosives. New. J. Chem. 2014, 38, 5754–5760. [Google Scholar] [CrossRef]
- Mehta, S.; Larock, S. Iodine/palladium approaches to the synthesis of polyheterocyclic compounds. J. Org. Chem. 2010, 75, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-B.; Adachi, Y.; Ooyama, Y.; Ohshita, J. Synthesis and properties of benzofuran-fused silole and germole derivatives: Reversible dimerization and crystal structures of monomers and dimers. Organometallics 2016, 35, 2327–2332. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Farinola, G.M.; Ragni, R. Electroluminescent materials for white organic light emitting diodes. Chem. Soc. Rev. 2011, 40, 3467–3482. [Google Scholar] [CrossRef] [PubMed]





| Compound | λabs a [nm] | ε [L·mol−1·cm−1] | Eopt b eV | λem c [nm] | ΦF d | E°ox e [V] | Epc f [V] | Td10 g [°C] |
|---|---|---|---|---|---|---|---|---|
| 2 | 360 | 19,000 | 3.12 | 429 | 0.75 | +0.72 | −2.73 | 203 |
| 4 | 368 | 19,000 | 3.03 | 441 | 0.86 | +0.79 | −2.63 | 290 |
| 5 | 368 | 17,000 | 3.03 | 440 | 0.68 | +0.78 | −2.68 | 318 |
| 6 | 369 | 18,000 | 3.03 | 443 | 0.76 | +0.79 | −2.66 | 385 |
| Device | Emitter | Doping Rate (%) | Von a (V) | EQE b (%) | CE b (cd/A) | PE b (Lm/W) | CIE Color Coordinates | Maximal Brightness (cd/m2) (@ mA/cm2) |
|---|---|---|---|---|---|---|---|---|
| A | 4 | pure | 3.8 | 1.7 | 2.4 | 1.1 | 0.16; 0.18 | 1040 (110) |
| B | 4 | 1 | 3.5 | 2.5 | 2.7 | 1.4 | 0.15; 0.15 | 1060 (110) |
| C | 4 | 4.5 | 3.5 | 2.4 | 2.8 | 1.4 | 0.15; 0.15 | 1580 (130) |
| D | 6 | pure | 4.3 | 0.5 | 0.9 | 0.3 | 0.20; 0.27 | 270 (90) |
| E | 6 | 1 | 3.9 | 1.3 | 1.3 | 0.6 | 0.18; 0.17 | 740 (140) |
| F | 6 | 4 | 3.4 | 1.2 | 1.5 | 0.7 | 0.17; 0.19 | 590 (120) |
| G | 6 | 6 | 3.6 | 1.1 | 1.5 | 0.7 | 0.18; 0.19 | 630 (150) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Denis, M.; Bouit, P.-A.; Zhang, Y.; Wei, X.; Tondelier, D.; Geffroy, B.; Duan, Z.; Hissler, M. Blue Electrofluorescence Properties of Furan–Silole Ladder Pi-Conjugated Systems. Appl. Sci. 2018, 8, 812. https://0-doi-org.brum.beds.ac.uk/10.3390/app8050812
Chen H, Denis M, Bouit P-A, Zhang Y, Wei X, Tondelier D, Geffroy B, Duan Z, Hissler M. Blue Electrofluorescence Properties of Furan–Silole Ladder Pi-Conjugated Systems. Applied Sciences. 2018; 8(5):812. https://0-doi-org.brum.beds.ac.uk/10.3390/app8050812
Chicago/Turabian StyleChen, Hui, Mathieu Denis, Pierre-Antoine Bouit, Yinlong Zhang, Xinda Wei, Denis Tondelier, Bernard Geffroy, Zheng Duan, and Muriel Hissler. 2018. "Blue Electrofluorescence Properties of Furan–Silole Ladder Pi-Conjugated Systems" Applied Sciences 8, no. 5: 812. https://0-doi-org.brum.beds.ac.uk/10.3390/app8050812