Flocculation Harvesting Techniques for Microalgae: A Review
Abstract
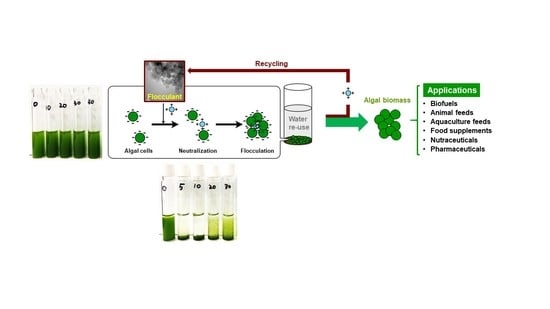
:1. Introduction
2. Auto-Flocculation
3. Bio-Flocculation
3.1. Algal–Fungal Bio-Flocculation
3.2. Algal–Yeast Bio-Flocculation
3.3. Algal–Bacterial Bio-Flocculation
3.4. Algal–Algal Bio-Flocculation
4. Chemical Flocculation
4.1. Inorganic Flocculant
4.2. Organic Flocculant
5. Particle-Based Flocculation
5.1. Aminoclay-Based Nanoparticle
5.2. Magnetic Particle
5.3. Multi-Functional Particle
6. Electrochemical Flocculation
6.1. Sacrificial Electrode
6.2. Non-Sacrificial Electrode
6.3. Electro-Flotation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Aminoclay |
| Al-AC | Aluminum aminoclay |
| APTES | 3-Aminopropyltriethoxysilane |
| Ca-AC | Calcium aminoclay |
| Ce-AC | Cerium aminoclay |
| ECF | Electrochemical flocculation |
| EOM | Extracellular organic matter |
| EPS | Extracellular polymeric substances |
| FAME | Fatty acid methyl ester |
| Fe-AC | Iron aminoclay |
| Mg-AC | Magnesium aminoclay |
| MNP | Magnetic nanoparticle |
| MW | Molecular weight |
| nZVI | Nanosacle zero-valent iron |
| PEI | Polyethylenimine |
| PLL | Poly-l-lysine |
| SA | Steric acid |
| SEM | Scanning electron microscopy |
References
- Chen, M.F.; Zhang, Y.Y.; Di He, M.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Qian, Z.J. Antioxidant peptide purified from enzymatic hydrolysates of Isochrysis Zhanjiangensis and its protective effect against ethanol induced oxidative stress of HepG2 cells. Biotechnol. Bioprocess Eng. 2019, 24, 308–317. [Google Scholar] [CrossRef]
- Eida, M.F.; Matter, I.A.; Darwesh, O.M. Cultivation of oleaginous microalgae Scenedesmus obliquus on secondary treated municipal wastewater as growth medium for biodiesel production. Ecol. Eng. 2018, 19, 38–50. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae-a review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Gad, M.S.; Abdo, S.M.; Abed, K.A.; Matter, I.A. Performance and exhaust emissions of a diesel engine burning algal biodiesel blends. Int. J. Mech. Eng. 2016, 16, 151–158. [Google Scholar]
- Choi, Y.Y.; Hong, M.E.; Chang, W.S.; Sim, S.J. Autotrophic biodiesel production from the thermotolerant microalga Chlorella sorokiniana by enhancing the carbon availability with temperature adjustment. Biotechnol. Bioprocess Eng. 2019, 24, 223–231. [Google Scholar] [CrossRef]
- Kim, B.; Praveenkumar, R.; Choi, E.; Lee, K.; Jeon, S.G.; Oh, Y.-K. Prospecting for oleaginous and robust Chlorella spp. for coal-fired flue-gas-mediated biodiesel production. Energies 2018, 11, 2026. [Google Scholar] [CrossRef]
- Branyikova, I.; Filipenska, M.; Urbanova, K.; Ruzicka, M.C.; Pivokonsky, M.; Branyik, T. Physicochemical approach to alkaline flocculation of Chlorella vulgaris induced by calcium phosphate precipitates. Colloids Surf. B Biointerfaces 2018, 166, 54–60. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lee, K.; Oh, Y.-K. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: A review. Bioresour. Technol. 2015, 184, 63–72. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.K. Influence of nitrogen source and growth phase on extracellular biosynthesis of silver nanoparticles using cultural filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting microalgae grown on wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar] [CrossRef]
- Matter, I.A.; Darwesh, O.M.; El-baz, F.K. Using the natural polymer chitosan in harvesting Scenedesmus species under different concentrations and cultural pH values. Int. J. Pharm. Biol. Sci. 2016, 7, 254–260. [Google Scholar]
- Rashid, N.; Park, W.K.; Selvaratnam, T. Binary culture of microalgae as an integrated approach for enhanced biomass and metabolites productivity, wastewater treatment, and bioflocculation. Chemosphere 2018, 194, 67–75. [Google Scholar] [CrossRef]
- Tiron, O.; Bumbac, C.; Manea, E.; Stefanescu, M.; Lazar, M.N. Overcoming microalgae harvesting barrier by activated algae granules. Sci. Rep. 2017, 7, 4646–4656. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. Integration of microalgal cultivation system for wastewater remediation and sustainable biomass production. World J. Microbiol. Biotechnol. 2016, 32, 139–149. [Google Scholar] [CrossRef]
- Son, J.; Sung, M.; Ryu, H.; Oh, Y.-K.; Han, J.-I. Microalgae dewatering based on forward osmosis employing proton exchange membrane. Bioresour. Technol. 2017, 244, 57–62. [Google Scholar] [CrossRef]
- Hwang, T.; Park, S.J.; Oh, Y.-K.; Rashid, N.; Han, J.I. Harvesting of Chlorella sp. KR-1 using a cross-flow membrane filtration system equipped with an anti-fouling membrane. Bioresour. Technol. 2013, 139, 379–382. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.; Lee, J. Optimization of cross flow filtration system for Dunaliella tertiolecta and Tetraselmis sp. microalgae harvest. Korean J. Chem. Eng. 2015, 32, 1377–1380. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation harvesting of microalgae. J. Renew. Sustain. Energy 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Garg, S.; Wang, L.; Schenk, P.M. Effective harvesting of low surface-hydrophobicity microalgae by froth flotation. Bioresour. Technol. 2014, 159, 437–441. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lee, A.K.; Kalaitzidis, T.; Ashman, P.J.; Sathe, S.; Lewis, D.M. Harvesting, thickening and dewatering microalgae biomass. In Algae for Biofuels and Energy; Borowwitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 5, pp. 165–185. ISBN 978-94-007-5478-2. [Google Scholar]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Alam, M.A.; Zhao, X.Q.; Zhang, X.Y.; Guo, S.L.; Ho, S.H.; Chang, J.S.; Bai, F.W. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.-Y.; Bui, V.K.H.; Oh, Y.-K.; Lee, Y.-C. Recent advanced applications of nanomaterials in microalgae biorefinery. Algal Res. 2019, 41, 101522. [Google Scholar] [CrossRef]
- Gultom, S.O.; Hu, B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 2013, 6, 5921–5939. [Google Scholar] [CrossRef]
- Alam, M.A.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Biotechnol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Microalgae autoflocculation: An alternative to high-energy consuming harvesting methods. J. Appl. Phycol. 2013, 25, 991–999. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Bakonyi, P.; Nemestóthy, N.; Xia, A.; Kumar, G. A review on chemical mechanism of microalgae flocculation via polymers. Biotechnol. Rep. 2019, 21, e00302. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Tao, Y.; Zhang, Y.; Li, A.; Li, T.; Sang, M.; Zhang, C. Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol. Biofuels 2013, 6, 98. [Google Scholar] [CrossRef]
- Shen, Y.; Cui, Y.; Yuan, W. Flocculation optimization of microalga Nannochloropsis oculata. Appl. Biochem. Biotechnol. 2013, 169, 2049–2063. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Uemura, Y.; Krishnan, V.; Khalid, N.A. Autoflocculation of Scenedesmus quadricauda: Effects of aeration rate, aeration gas CO2 concentration and medium nitrogen concentration. Int. J. Biomass Renew. 2017, 6, 11–17. [Google Scholar]
- Huo, S.; Wang, Z.; Zhu, S.; Cui, F.; Zou, B.; You, W.; Yuan, Z.; Dong, R. Optimization of alkaline flocculation for harvesting of Scenedesmus quadricauda #507 and Chaetoceros muelleri #862. Energies 2014, 7, 6186–6195. [Google Scholar]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef]
- Yoo, C.; La, H.J.; Kim, S.C.; Oh, H.M. Simple processes for optimized growth and harvest of Ettlia sp. by pH control using CO2 and light irradiation. Biotechnol. Bioeng. 2015, 112, 288–296. [Google Scholar] [CrossRef]
- Tran, N.A.T.; Seymour, J.R.; Siboni, N.; Evenhuis, C.R.; Tamburic, B. Photosynthetic carbon uptake induces autoflocculation of the marine microalga Nannochloropsis oculata. Algal Res. 2017, 26, 302–311. [Google Scholar] [CrossRef]
- Guo, S.L.; Zhao, X.Q.; Wan, C.; Huang, Z.Y.; Yang, Y.L.; Alam, M.A.; Ho, S.H.; Bai, F.W.; Chang, J.S. Characterization of flocculating agent from the self-flocculating microalga Scenedesmus obliquus AS-6-1 for efficient biomass harvest. Bioresour. Technol. 2013, 145, 285–289. [Google Scholar] [CrossRef]
- Matter, I.A.; Darwesh, O.M.; Eida, M.F. Harvesting of microalgae Scenedesmus obliquus using chitosan-alginate dual flocculation system. Biosci. Res. 2018, 15, 540–548. [Google Scholar]
- Brady, P.V.; Pohl, P.I.; Hewson, J.C. A coordination chemistry model of algal autoflocculation. Algal Res. 2014, 5, 226–230. [Google Scholar] [CrossRef]
- Lv, J.; Wang, X.; Liu, W.; Feng, J.; Liu, Q.; Nan, F.; Jiao, X.; Xie, S. The performance of a self-flocculating microalga Chlorococcum sp. GD in wastewater with different ammonia concentrations. Int. J. Environ. Res. Public Health 2018, 15, 434. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Van Den Hende, S.; Vervaeren, H.; Coward, T.; Skill, S.C. Harvesting of microalgae within a biorefinery approach: A review of the developments and case studies from pilot-plants. Algal Res. 2015, 11, 248–262. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Frappart, M.; Jaouen, P.; Pruvost, J.; Bourseau, P. Harvesting Chlorella vulgaris by natural increase in pH: Effect of medium composition. Environ. Technol. 2014, 35, 1378–1388. [Google Scholar] [CrossRef]
- Prochazkova, G.; Kastanek, P.; Branyik, T. Harvesting freshwater Chlorella vulgaris with flocculant derived from spent brewer’s yeast. Bioresour. Technol. 2015, 177, 28–33. [Google Scholar] [CrossRef]
- Salim, S.; Kosterink, N.R.; Wacka, N.T.; Vermuë, M.H.; Wijffels, R.H. Mechanism behind autoflocculation of unicellular green microalgae Ettlia texensis. J. Biotechnol. 2014, 174, 34–38. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Kumar, P.; Malik, A.; Choudhary, P. Exploring pellet forming filamentous fungi as tool for harvesting non-flocculating unicellular microalgae. Bioenergy Res. 2014, 7, 1430–1440. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A comparative study between fungal pellet-and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef]
- Díaz-Santos, E.; Vila, M.; de la Vega, M.; León, R.; Vigara, J. Study of bioflocculation induced by Saccharomyces bayanus var. uvarum and flocculating protein factors in microalgae. Algal Res. 2015, 8, 23–29. [Google Scholar]
- Lee, J.; Cho, D.H.; Ramanan, R.; Kim, B.H.; Oh, H.M.; Kim, H.S. Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour. Technol. 2013, 131, 195–201. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, X.Q.; Guo, S.L.; Alam, M.A.; Bai, F.W. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour. Technol. 2013, 135, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, B.; Gálvez, J.Á.; Michel, M.; Bartual, A. Microalgae cultivation in urban wastewater: Coelastrum cf. Pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 2017, 228, 210–217. [Google Scholar]
- Yen, H.W.; Chen, P.W.; Chen, L.J. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour. Technol. 2015, 184, 148–152. [Google Scholar] [CrossRef]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Co-culture for lipid production: Advances and challenges. Biomass Bioenergy 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Kim, B.; Praveenkumar, R.; Lee, J.; Nam, B.; Kim, D.M.; Lee, K.; Lee, Y.C.; Oh, Y.-K. Magnesium aminoclay enhances lipid production of mixotrophic Chlorella sp. KR-1 while reducing bacterial populations. Bioresour. Technol. 2016, 219, 608–613. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tao, Y.; Wu, J.; Zhu, Y.; Gao, B.; Tang, Y.; Li, A.; Zhang, C.; Zhang, Y. Effective flocculation of target microalgae with self-flocculating microalgae induced by pH decrease. Bioresour. Technol. 2014, 167, 367–375. [Google Scholar] [CrossRef]
- Yaser, A.Z.; Cassey, T.L.; Hairul, M.A.; Shazwan, A.S. Current review on the coagulation/flocculation of lignin containing wastewater. Int. J. Waste Res. 2014, 4, 153–159. [Google Scholar]
- Toh, P.Y.; Azenan, N.F.; Wong, L.; Ng, Y.S.; Chng, L.M.; Lim, J.; Chan, D.J.C. The role of cationic coagulant-to-cell interaction in dictating the flocculation-aided sedimentation of freshwater microalgae. Arab. J. Sci. Eng. 2017, 43, 1–9. [Google Scholar] [CrossRef]
- Kim, D.G.; Oh, H.M.; Park, Y.H.; Kim, H.S.; Lee, H.G.; Ahn, C.Y. Optimization of flocculation conditions for Botryococcus braunii using response surface methodology. J. Appl. Phycol. 2013, 25, 875–882. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Lu, M.; Lee, E.Y.; Lee, J. Harvesting of microalgae using flocculation combined with dissolved air flotation. Biotechnol. Bioprocess Eng. 2014, 19, 143–149. [Google Scholar] [CrossRef]
- Sanyano, N.; Chetpattananondh, P.; Chongkhong, S. Coagulation–flocculation of marine Chlorella sp. for biodiesel production. Bioresour. Technol. 2013, 147, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Gerde, J.A.; Yao, L.; Lio, J.; Wen, Z.; Wang, T. Microalgae flocculation: Impact of flocculant type, algae species and cell concentration. Algal Res. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, H.; Zhou, W.; Liu, Y.; Chen, P.; Ruan, R. Enhanced harvesting of Chlorella vulgaris using combined flocculants. Appl. Biochem. Biotechnol. 2016, 180, 791–804. [Google Scholar] [CrossRef]
- Choi, H.J. Effect of eggshells for the harvesting of microalgae species. Biotechnol. Biotechnol. Equip. 2015, 29, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Surendhiran, D.; Vijay, M. Study on flocculation efficiency for harvesting Nannochloropsis oculata for biodiesel production. Int. J. ChemTech Res. 2013, 5, 1761–1769. [Google Scholar]
- Kim, D.Y.; Lee, K.; Lee, J.; Lee, Y.H.; Han, J.I.; Park, J.Y.; Oh, Y.-K. Acidified-flocculation process for harvesting of microalgae: Coagulant reutilization and metal-free-microalgae recovery. Bioresour. Technol. 2017, 239, 190–196. [Google Scholar] [CrossRef]
- Vandamme, D.; Beuckels, A.; Markou, G.; Foubert, I.; Muylaert, K. Reversible flocculation of microalgae using magnesium hydroxide. Bioenergy Res. 2015, 8, 716–725. [Google Scholar] [CrossRef]
- Rahul, R.; Kumar, S.; Jha, U.; Sen, G. Cationic inulin: A plant based natural biopolymer for algal biomass harvesting. Int. J. Biol. Macromol. 2015, 72, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Hansel, P.A.; Riefler, R.G.; Stuart, B.J. Efficient flocculation of microalgae for biomass production using cationic starch. Algal Res. 2014, 5, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Anthony, R.; Sims, R. Cationic starch for microalgae and total phosphorus removal from wastewater. J. Appl. Polym. Sci. Symp. 2013, 130, 2572–2578. [Google Scholar] [CrossRef]
- Peng, C.; Li, S.; Zheng, J.; Huang, S.; Li, D. Harvesting microalgae with different sources of starch-based cationic flocculants. Appl. Biochem. Biotechnol. 2017, 181, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Tork, M.B.; Khalilzadeh, R.; Kouchakzadeh, H. Efficient harvesting of marine Chlorella vulgaris microalgae utilizing cationic starch nanoparticles by response surface methodology. Bioresour. Technol. 2017, 243, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Rehman, S.U.; Han, J.I. Rapid harvesting of freshwater microalgae using chitosan. Process Biochem. 2013, 48, 1107–1110. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, M.; Guldhe, A.; Ansari, F.A.; Rawat, I.; Kanney, K.; Bux, F. Design and development of polyamine polymer for harvesting microalgae for biofuels production. Energy Convers. Manag. 2014, 85, 537–544. [Google Scholar] [CrossRef]
- Wang, L.; Liang, W.; Yu, J.; Liang, Z.; Ruan, L.; Zhang, Y. Flocculation of Microcystis aeruginosa using modified larch tannin. Environ. Sci. Technol. 2013, 47, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Selesu, N.F.; de Oliveira, V.T.; Corrêa, D.O.; Miyawaki, B.; Mariano, A.B.; Vargas, J.V.; Vieira, R.B. Maximum microalgae biomass harvesting via flocculation in large scale photobioreactor cultivation. Can. J. Chem. Eng. 2016, 94, 304–309. [Google Scholar] [CrossRef]
- Roselet, F.; Burkert, J.; Abreu, P.C. Flocculation of nannochloropsis oculata using a tannin-based polymer: Bench scale optimization and pilot scale reproducibility. Biomass Bioenerg. 2016, 87, 55–60. [Google Scholar] [CrossRef]
- Noh, W.; Kim, J.; Lee, S.J.; Ryu, B.G.; Kang, C.M. Harvesting and contamination control of microalgae Chlorella ellipsoidea using the bio-polymeric flocculant α-poly-l-lysine. Bioresour. Technol. 2018, 249, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Al Hattab, M.; Ghaly, A.; Hammouda, A. Microalgae harvesting methods for industrial production of biodiesel: Critical review and comparative analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 154. [Google Scholar] [CrossRef]
- Martínez, T.D.C.C.; Rodríguez, R.A.; Voltolina, D.; Morquecho, L. Effectiveness of coagulants-flocculants for removing cells and toxins of Gymnodinium catenatum. Aquaculture 2016, 452, 188–193. [Google Scholar] [CrossRef]
- Fast, S.A.; Kokabian, B.; Gude, V.G. Chitosan enhanced coagulation of algal turbid waters–comparison between rapid mix and ultrasound coagulation methods. Chem. Eng. J. 2014, 244, 403–410. [Google Scholar] [CrossRef]
- Rehn, G.; Grey, C.; Branneby, C.; Adlercreutz, P. Chitosan flocculation: An effective method for immobilization of E. coli for biocatalytic processes. J. Biotechnol. 2013, 165, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.J.; Ellis, J.T.; Sathish, A.; Rahman, A.; Miller, C.D.; Sims, R.C. Effect of coagulant/flocculants on bioproducts from microalgae. Bioresour. Technol. 2013, 149, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Phang, S.M.; Juan, J.C.; Ramanan, R.N. Separation of Chlorella biomass from culture medium by flocculation with rice starch. Algal Res. 2018, 30, 162–172. [Google Scholar] [CrossRef]
- Facchi, D.P.; Lima, A.C.; de Oliveira, J.H.; Lazarin-Bidóia, D.; Nakamura, C.V.; Canesin, E.A.; Bonafé, E.G.; Monteiro, J.P.; Visentainer, J.V.; Muniz, E.C.; et al. Polyelectrolyte complexes based on alginate/tanfloc: Optimization, characterization and medical application. Int. J. Biol. Macromol. 2017, 103, 129–138. [Google Scholar] [CrossRef]
- Chheda, A.H.; Vernekar, M.R. A natural preservative ε-poly-l-lysine: Fermentative production and applications in food industry. Int. Food Res. J. 2015, 22, 23–30. [Google Scholar]
- Zheng, Z.; Wang, R.; Yao, H.; Xie, S.; Zhang, Y.; Hou, J.; Zhou, H.; Tang, Z. Polyamino acid interlayer facilitates electron extraction in narrow band gap fullerene-free organic solar cells with an outstanding short-circuit current. Nano Energy 2018, 50, 169–175. [Google Scholar] [CrossRef]
- Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antimicrobial action of ε-poly-l-lysine. J. Antibiot. 1984, 37, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, T.; Liimatainen, H.; Hormi, O.; Niinimäki, J. Coagulation–flocculation treatment of municipal wastewater based on anionized nanocelluloses. Chem. Eng. J. 2013, 231, 59–67. [Google Scholar] [CrossRef]
- Datta, K.; Achari, A.; Eswaramoorthy, M. Aminoclay: A functional layered material with multifaceted applications. J. Mater. Chem. A 2013, 1, 6707–6718. [Google Scholar] [CrossRef]
- Lee, Y.C.; Park, W.K.; Yang, J.W. Removal of anionic metals by amino-organoclay for water treatment. J. Hazard. Mater. 2011, 190, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lee, Y.C.; Park, S.B.; Han, J.I. Gaseous carbon dioxide conversion and calcium carbonate preparation by magnesium phyllosilicate. RSC Adv. 2014, 4, 4037–4040. [Google Scholar] [CrossRef]
- Farooq, W.; Lee, Y.C.; Han, J.I.; Darpito, C.H.; Choi, M.; Yang, J.W. Efficient microalgae harvesting by organo-building blocks of nanoclays. Green Chem. 2013, 15, 749–755. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, B.; Farooq, W.; Chung, J.; Han, J.I.; Shin, H.J.; Jeong, S.H.; Park, J.Y.; Lee, J.S.; Oh, Y.-K. Harvesting of oleaginous Chlorella sp. by organoclays. Bioresour. Technol. 2013, 132, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, H.U.; Lee, K.; Kim, B.; Lee, S.Y.; Choi, M.H.; Farooq, W.; Choi, J.S.; Park, J.Y.; Lee, J.; et al. Aminoclay-conjugated TiO2 synthesis for simultaneous harvesting and wet-disruption of oleaginous Chlorella sp. Chem. Eng. J. 2014, 245, 143–149. [Google Scholar] [CrossRef]
- Lee, Y.C.; Oh, S.Y.; Lee, H.U.; Kim, B.; Lee, S.Y.; Choi, M.H.; Lee, G.W.; Park, J.Y.; Oh, Y.-K.; Ryu, T.; et al. Aminoclay-induced humic acid flocculation for efficient harvesting of oleaginous Chlorella sp. Bioresour. Technol. 2014, 153, 365–369. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, K.; Hwang, Y.; Andersen, H.R.; Kim, B.; Lee, S.Y.; Choi, M.H.; Park, J.Y.; Han, Y.K.; Oh, Y.-K.; et al. Aminoclay-templated nanoscale zero-valent iron (nZVI) synthesis for efficient harvesting of oleaginous microalga, Chlorella sp. KR-1. RSC Adv. 2014, 4, 4122–4127. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, K.; Lee, S.Y.; Jeon, S.G.; Na, J.G.; Oh, Y.-K.; Park, S.B. Effect of barium ferrite particle size on detachment efficiency in magnetophoretic harvesting of oleaginous Chlorella sp. Bioresour. Technol. 2014, 152, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bui, V.K.H.; Farooq, W.; Jeon, S.G.; Oh, Y.-K.; Lee, Y.C. Magnesium aminoclay-Fe3O4 (MgAC-Fe3O4) hybrid composites for harvesting of mixed microalgae. Energies 2018, 11, 1359. [Google Scholar] [CrossRef]
- Ji, H.M.; Lee, H.U.; Kim, E.J.; Seo, S.; Kim, B.; Lee, G.W.; Oh, Y.-K.; Kim, J.Y.; Huh, Y.S.; Song, H.A.; et al. Efficient harvesting of wet blue-green microalgal biomass by two-aminoclay [AC]-mixture systems. Bioresour. Technol. 2016, 15, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, C.; Wang, F.; Zheng, S.; Liu, C.Z. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour. Technol. 2011, 102, 10047–10051. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.R.; Wang, F.; Wang, S.K.; Liu, C.Z.; Guo, C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.Y.; Praveenkumar, R.; Kim, B.; Seo, J.Y.; Jeon, S.G.; Na, J.G.; Park, J.Y.; Kim, D.M.; Oh, Y.-K. Repeated use of stable magnetic flocculant for efficient harvest of oleaginous Chlorella sp. Bioresour. Technol. 2014, 167, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Lee, K.; Praveenkumar, R.; Kim, B.; Lee, S.Y.; Oh, Y.-K.; Park, S.B. Tri-functionality of Fe3O4-embedded carbon microparticles in microalgae harvesting. Chem. Eng. J. 2015, 2015, 206–214. [Google Scholar] [CrossRef]
- Hu, Y.R.; Guo, C.; Wang, F.; Wang, S.K.; Pan, F.; Liu, C.Z. Improvement of microalgae harvesting by magnetic nanocomposites coated with polyethylenimine. Chem. Eng. J. 2014, 242, 341–347. [Google Scholar] [CrossRef]
- Ge, S.; Agbakpe, M.; Wu, Z.; Kuang, L.; Zhang, W.; Wang, X. Influences of surface coating, UV irradiation and magnetic field on the algae removal using magnetite nanoparticles. Environ. Sci. Technol. 2015, 49, 1190–1196. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.Y.; Na, J.G.; Jeon, S.G.; Praveenkumar, R.; Kim, D.M.; Chang, W.S.; Oh, Y.-K. Magnetophoretic harvesting of oleaginous Chlorella sp. by using biocompatible chitosan/magnetic nanoparticle composites. Bioresour. Technol. 2013, 149, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Paul, A.; Gangopadhyay, M.; Singh, N.P.; Sen, R. Smart and reusable biopolymer nanocomposite for simultaneous microalgal biomass harvesting and disruption: Integrated downstream processing for a sustainable biorefinery. ACS Sustain. Chem. Eng. 2016, 5, 852–861. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.C. Aminoclays for biological and environmental applications: An updated review. Chem. Eng. J. 2018, 336, 757–772. [Google Scholar] [CrossRef]
- Wells, J.; Kazakova, O.; Posth, O.; Steinhoff, U.; Petronis, S.; Bogart, L.K.; Southern, P.; Pankhurst, Q.; Johansson, C. Standardisation of magnetic nanoparticles in liquid suspension. J. Phys. D Appl. Phys. 2017, 50, 383003. [Google Scholar] [CrossRef]
- Bitton, G.; Fox, J.L.; Strickland, H.G. Removal of algae from Florida lakes by magnetic filtration. Appl. Microbiol. 1975, 30, 905–908. [Google Scholar]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Harvesting microalgae by magnetic separation: A review. Algal Res. 2015, 9, 175–185. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Yen, H.W.; Ho, S.H.; Lo, Y.C.; Cheng, C.L.; Ren, N.; Chang, J.S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef]
- Zhu, L.D.; Hiltunen, E.; Li, Z. Using magnetic materials to harvest microalgal biomass: Evaluation of harvesting and detachment efficiency. Environ. Technol. 2019, 40, 1006–1012. [Google Scholar] [CrossRef]
- Toh, P.Y.; Ng, B.W.; Ahmad, A.L.; Chieh, D.C.J.; Lim, J. Magnetophoretic separation of Chlorella sp.: Role of cationic polymer binder. Process Saf. Environ. Prot. 2014, 92, 515–521. [Google Scholar] [CrossRef]
- Ge, S.; Agbakpe, M.; Zhang, W.; Kuang, L.; Wu, Z.; Wang, X. Recovering magnetic Fe3O4–ZnO nanocomposites from algal biomass based on hydrophobicity shift under UV irradiation. ACS Appl. Mater. Interfaces 2015, 7, 11677–11682. [Google Scholar] [CrossRef]
- Seo, J.Y.; Kim, M.G.; Lee, K.; Lee, Y.C.; Na, J.G.; Jeon, S.G.; Park, S.B.; Oh, Y.-K. Multifunctional Nanoparticle Applications to Microalgal Biorefinery. In Nanotechnology for Bioenergy and Biofuel Production, 1st ed.; Rai, M., da Silva, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 59–87. ISBN 978-3-319-45458-0. [Google Scholar]
- Chiang, Y.D.; Dutta, S.; Chen, C.T.; Huang, Y.T.; Lin, K.S.; Wu, J.C.; Suzuki, N.; Yamauchi, Y.; Wu, K.C.W. Functionalized Fe3O4@silica core-shell nanoparticles as microalgae harvester and catalyst for biodiesel production. ChemSusChem 2015, 8, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Guldhe, A.; Singh, P.; Rawat, I.; Bux, F. Electrochemical harvesting process for microalgae by using nonsacrificial carbon electrode: A sustainable approach for biodiesel production. Chem. Eng. J. 2014, 255, 327–333. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Rahmani, A.; Zerrouki, D.; Djafer, L.; Ayral, A. Hydrogen recovery from the photovoltaic electroflocculation-flotation process for harvesting Chlorella pyrenoidosa microalgae. Int. J. Hydrog. Energy 2017, 42, 19591–19596. [Google Scholar] [CrossRef]
- Fayad, N.; Yehya, T.; Audonnet, F.; Vial, C. Harvesting of microalgae Chlorella vulgaris using electro-coagulation-flocculation in the batch mode. Algal Res. 2017, 25, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Hajar, H.A.A.; Riefler, G.; Stuart, B.J. Investigation of electrolytic flocculation for microalga Scenedesmus sp. using aluminum and graphite electrodes. RSC Adv. 2018, 8, 38808–38817. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, L.; Chen, Q.; Lu, J.; Pan, G.; Hu, L.; Yi, Q. Synergy of flocculation and flotation for microalgae harvesting using aluminium electrolysis. Bioresour. Technol. 2017, 233, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-coagulation–flotation process for algae removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.T.; Santos, M.; Nobre, B.P.; Gouveia, L. Nannochloropsis sp. biomass recovery by electro-coagulation for biodiesel and pigment production. Bioresour. Technol. 2013, 134, 219–226. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes Sandra Cláudia, V.; Goiris, K.; Foubert, I.; Pinoy Luc Jozef, J.; Muylaert, K. Evaluation of electro-coagulation-flocculation for harvesting marine and freshwater microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [Green Version]
- Uduman, N.; Bourniquel, V.; Danquah, M.K.; Hoadley, A.F.A. A parametric study of electrocoagulation as a recovery process of marine microalgae for biodiesel production. Chem. Eng. J. 2011, 174, 249–257. [Google Scholar] [CrossRef]
- Valero, E.; Álvarez, X.; Cancela, Á.; Sánchez, Á. Harvesting green algae from eutrophic reservoir by electroflocculation and post-use for biodiesel production. Bioresour. Technol. 2015, 187, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cañizares, P.; Martínez, F.; Jiménez, C.; Lobato, J.; Rodrigo, M.A. Coagulation and electrocoagulation of wastes polluted with dyes. Environ. Sci. Technol. 2006, 40, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Emamjomeh, M.M.; Sivakumar, M. Fluoride removal by a continuous flow electrocoagulation reactor. J. Environ. Manag. 2009, 90, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, L.; Cheng, W.; Chen, L.; Wang, J.; Wang, H.; Zhang, W.; Liu, T. Electro-flotation of Chlorella sp. Assisted with flocculation by chitosan. Algal Res. 2016, 18, 7–14. [Google Scholar] [CrossRef]
- Shin, H.; Kim, K.; Jung, J.Y.; Bai, S.C.; Chang, Y.K.; Han, J.I. Harvesting of Scenedesmus obliquus cultivated in seawater using electro-flotation. Korean J. Chem. Eng. 2017, 34, 62–65. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, J.; Zhao, B.; Zhu, P. Microbubble size distribution measurement in a DAF system. Ind. Eng. Chem. Res. 2015, 54, 5179–5183. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, B.G.; Kim, B.K.; Han, J.I.; Yang, J.W. Continuous microalgae recovery using electrolysis with polarity exchange. Bioresour. Technol. 2012, 111, 268–275. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, B.G.; Kim, K.; Kim, B.K.; Han, J.I.; Yang, J.W. Continuous microalgae recovery using electrolysis: Effect of different electrode pairs and timing of polarity exchange. Bioresour. Technol. 2012, 123, 164–170. [Google Scholar] [CrossRef]
- Sing, S.F.; Isdepsky, A.; Borowitzka, M.A.; Lewis, D.M. Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. In raceway ponds under increasing salinity: A novel protocol for commercial microalgal biomass production. Bioresour. Technol. 2014, 161, 47–54. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]







| Condition | Alga (Cell Density) | Optimal Harvesting | Ref. | |
|---|---|---|---|---|
| Acidic pH | pH 4.0 | C. ellipsoideum (4.38 g/L) | 95% @ 15 min | [31] |
| pH 4.0 | C. nivale (4.17 g/L) | 94% @ 15 min | [31] | |
| pH 4.0 | Scenedesmus sp. (6.94 g/L) | 98% @ 15 min | [31] | |
| Alkaline pH | pH 11.5 | C. muelleri #862 (0.42 g/L) | 100% @ 30 min | [34] |
| pH 11.0 | C. vulgaris (0.5 g/L) | 95% @ 60 min | [35] | |
| pH 12.0 | Chlorococcum sp. R-AP13 | 94% @ 10 min | [36] | |
| pH 12.5 | Ettlia sp. YC001 (1.2 g/L) | 94% @ 30 min | [37] | |
| pH 10.4 | N. oculate (2.27 × 105 cells/mL) | 90% @ 10 min | [38] | |
| pH 11.6 | S. quadricauda #507 (0.54 g/L) | 95% @ 30 min | [34] | |
| Culture aging | 16 days | S. obliquus AS-6-1 (2.25 g/L) | 80% @ 30 min | [39] |
| Flocculant (Dosage) | Alga (Cell Density, Volume or Amount) | Optimal Harvesting | Ref. | |
|---|---|---|---|---|
| Fungus | A. fumigatus | C. protothecoides | ~90% @ 24 h | [47] |
| A. fumigatus (1.5–2.0 × 107 spores/L) | S. quadricauda (5–8 × 108 cell/mL) | ~97% @ 48 h | [49] | |
| A. fumigatus | T. suecica | ~90% @ 24 h | [47] | |
| A. lentulus (1.0 × 106 spores/mL) | Chroococcus sp. (1.58 g/L) | ~100% @ 24 h | [48] | |
| Penicillium cells (1.92 g) | Chlorella sp. (3.84 g) | ~98% @ 2.5 h | [50] | |
| Penicillium spores (1.1 × 104 cells/mL) | Chlorella sp. (3.84 g) | ~99% @ 28 h | [50] | |
| Yeast | Extracellular protein of S. bayanus (0.1 g/L) | C. reinhardtii (10 mL) | 95% @ 3 h | [51] |
| Extracellular protein of S. bayanus (0.1 g/L) | Picochlorum sp. (10 mL) | 75% @ 3 h | [51] | |
| S. bayanus (1:1, v/v) | C. reinhardtii (10 mL) | 80% @ 6 h | [51] | |
| S. bayanus (1:1, v/v) | Picochlorum sp. (10 mL) | 60% @ 6 h | [51] | |
| S. pastorianus (0.4 mg/g cell) | C. vulgaris (5 g/L) | 90% @ 70 min | [45] | |
| Bacterium | Flavobacterium, Terrimonas, and Sphingobacterium | C. vulgaris (6 × 106 cells/mL) | 94% @ 24 h | [52] |
| Bio-flocculant secreted from S. silvestris W01 (3:1, w/w) | N. oceanica DUT01 | 90% @ 10 min | [53] | |
| Alga | S. obliquus AS-6–1 (1%, v/v) | S. obliquus FSP-3 (10 mL) | 83% @ 30 min | [39] |
| Exudates-rich spent media of C. cf. pseudomicroporum (1:1, v/v) | S. ellipsoideus (15 mL) | 97% @ 4 h | [54] | |
| Phormidium sp. | Chlorella sp. | 100% @ 5 min | [14] | |
| Flocculant (Dosage) | Alga (Cell Density or Volume) | Optimal Harvesting | Ref. | |
|---|---|---|---|---|
| Inorganic flocculant | Al2(SO4)3 (1.2 g/L) | Tetraselmis sp. KCTC12236BP (3 g/L) | 86% @ 30 min | [64] |
| Al2(SO4)3 (152 mg/L) | Chlorella sp. (0.12 g/L) | 100% @ 60 min | [65] | |
| Al2(SO4)3 (180 mg/L) | Scenedesmus sp. (0.20 g/L) | 90% @ 20 min | [66] | |
| Al2(SO4)3 (20 mg/L) | C. reinhardtii (0.31 g/L) | 90% @ 20 min | [66] | |
| Al2(SO4)3 (438.1 μM) | N. oculata (1.7 g/L) | 92% @ 320 min | [32] | |
| Al2(SO4)3 (50 mg/L) | S. limacinum (0.93 g/L) | 90% @ 20 min | [66] | |
| CaO (60 mg/L) | C. vulgaris (1.5 g/L) | 85% @ 5min | [67] | |
| CaCO3-rich eggshell (80 mg/L) | C. vulgaris (2.3 g/L) | 99% @ 20 min | [68] | |
| FeCl3 (0.4 g/L) | N. oculata (50 mL) | 94% @ 180 min | [69] | |
| FeCl3 (143 mg/L) | Chlorella sp. (0.12 g/L) | 100% @ 40 min | [65] | |
| FeCl3 (438.1 μM) | N. oculata (2.2 g/L) | 78% @ 320 min | [32] | |
| Fe2(SO4)3 (0.6 g/L) | N. oculata (50 mL) | 87% @ 180 min | [69] | |
| Fe2(SO4)3 (1.0 g/L) | Chlorella sp. KR-1 (1.52 g/L) | 98% @ 30 min | [70] | |
| Mg(OH)2 (1 mM) | Chlorella sp. (0.1 g/L) | 90% @ 30 min | [71] | |
| Organic flocculant | Cationic inulin (60 mg/L) | Botryococcus sp. | 89% @ 15 min | [72] |
| Cationic starches (0.01 g/L) | S. dimorphus (0.12 g/L) | 95% @ 90 min | [73] | |
| Cationic starches (1.4:1, w/w) | S. obliquus | 90% @ 60 min | [74] | |
| Cationic starches (119 mg/g cell) | B. braunii (0.62 g/L) | 94% @ 20 min | [75] | |
| Cationic starches (50 mg/L) | S. limacinum (0.93 g/L) | 90% @ 20 min | [66] | |
| Cationic starches (7.1 mg/L) | C. vulgaris (0.75 g/L) | 90% @ 120 min | [76] | |
| Cationic starches (89 mg/g cell) | C. pyrenoidosa (1.02 g/L) | 96% @ 20 min | [75] | |
| Chitosan (10 mg/g cell) | C. sorokiniana | 99% @ 45 min | [77] | |
| Chitosan (120 mg/L)) | C. vulgaris (1 g/L) | 99% @ 3 min | [78] | |
| Chitosan (40 mg/L) | Scenedesmus sp. A1 | 82% @ 60 min | [12] | |
| Chitosan (30 mg/L) | Chlorella sp. (3 × 107 cells/mL) | 97% @ 60 min | [61] | |
| Chitosan (30 mg/L) + sodium alginate (40 mg/L) | S. obliquus | 86% @ 60 min | [40] | |
| Epichlorohydrin-n,n- diisopropylamine-dimethylamine (8 mg/L) | Scenedesmus sp. (100 mL) | >90% @ 30 min | [79] | |
| Modified tannin (10 mg/L) | M. aeruginosa (1 × 109 cells/L) | 97% @ 120 min | [80] | |
| Modified tannin (210 mg/L) | Scenedesmus sp. | 97% @ 40 min | [81] | |
| Modified tannin (10 mg/L) | N. oculate (400 mg/L) | 98% @ 30 min | [82] | |
| Poly-L-lysine (70–150 kDa, 0.5 mg/L) | C. ellipsoidea (1 g/L) | 98% @ 75 min | [83] | |
| Kind | Flocculant | Dosage | Alga (Cell Density) | Optimal Harvesting | Ref. |
|---|---|---|---|---|---|
| Aminoclay-based nanoparticle | Al-AC | 0.6 g/L | Chlorella sp. KR-1 (1.7 g/L) | 100% @ 30 min | [99] |
| AC-conjugated TiO2 | 3.0 g/L | Chlorella sp. KR-1 (1.5 g/L) | ~85% @ 10 min | [100] | |
| AC-induced humic acid | 5.0 g/L | Chlorella sp. (1.3 g/L) | ~100% @ 30 min | [101] | |
| AC-templated nZVI | 19.1 g/L | Chlorella sp. KR-1 (1.5 g/L) | ~100% @ 3 min | [102] | |
| APTES-coated BaFe12O19 | 2.3 g/g cell | Chlorella sp. KR-1 | 99% @ 3 min | [103] | |
| MgAC-Fe3O4 hybrid composites | 4.7 g/L | Chlorella sp. KR-1 (1.75 g/L) | 99% @ 10 min | [104] | |
| MgAC-Fe3O4 hybrid composites | 4.3 g/L | S. obliquus (2.0 g/L) | 99% @ 10 min | [104] | |
| Mg-APTES | 0.6 g/L | Chlorella sp. KR-1 (1.7 g/L) | 100% @ 30 min | [99] | |
| Mg-APTES | 1.0 g/L | C. vulgaris (1.0 g/L) | 97% @ 125 min | [98] | |
| Mg-AC and Ce-AC | 0.2 g/L | Cyanobacteria | ~100% @ 60 min | [105] | |
| Magnetic particle | Fe3O4 nanoparticle | 55.9 mg cell/mg particles | B. braunii | 98% @ 1 min | [106] |
| Fe3O4 nanoparticle | 5.8 mg cell/mg particles | C. ellipsoidea | 98% @ 1 min | [106] | |
| Fe3O4 nanoparticle | 0.12 g/L | N. maritima | 95% @ 4 min | [107] | |
| Fe3O4 magnetic particle | 10 g/g cell | Chlorella sp. KR-1 | 99% @ 1 min | [108] | |
| Fe3O4-embedded carbon microparticle | ~25 g/L | Chlorella sp. KR-1 (~2 g/L) | 99% @ 1 min | [109] | |
| Fe3O4–PEI nanocomposite | 0.02 g/L | C. ellipsoidea (0.75 g/L) | 97% @ 2 min | [110] | |
| PEI-coated Fe3O4 | 0.2 g/L | S. dimorphus (1.8 g/L) | 82.7% @ 3 min | [111] | |
| Fe3O4-carbon-microparticle | 10 g/L | Chlorella sp. KR-1 (2.0 g/L) | 99% @ 1 min | [109] | |
| Chitosan–Fe3O4 composite | 1.4 g/L | Chlorella sp. KR-1 (1.0 g/L) | ~99% @ 5 min | [112] | |
| Chitosan-coated Fe3O4-TiO2 | 0.07 g/g cell | C. minutissima (3.0 g/L) | 98% @ 2 min | [113] |
| Electrode | Alga (Cell Density) | Optimal Harvesting (Energy Requirement) | Ref. | |
|---|---|---|---|---|
| Sacrificial | Al | C. pyrenoidosa | 95.8% @ 1 min (0.3 kWh/kg cell) | [126] |
| Al | C. vulgaris | 98% @ 4 min (0.3 kWh/kg cell) | [129] | |
| Al | M. aeruginosa (1.3 × 109 cells/mL) | 100% @ 45 min (0.4 kWh/m3) | [130] | |
| Al | Nannochloropsis sp. (2.5 g/L) | 97% @ 10 min (0.06 kWh/kg cell) | [131] | |
| Al | Scenedesmus sp. | ~98.5% @ 20 min (2.3 kWh/kg cell) | [128] | |
| Al | P. tricornutum | 80% @ 30 min (0.2 kWh/kg cell) | [132] | |
| Fe | C. vulgaris | 80% @ 30 min (2.1 kWh/kg cell) | [132] | |
| Fe | Chlorococcum sp. | 96% @ 15 min (9.2 kWh/kg cell) | [133] | |
| Fe | Green algae mixture (Scenedesmus, Kirchneriella, and Microcystis) | ~95.6% @ 24 h (4.4 kWh/kg cell) | [134] | |
| Fe | Tetraselmis sp. | 94% @ 15 min (4.4 kWh/kg cell) | [133] | |
| Non-sacrificial | C | C. sorokiniana | ~95% @ 15 min (1.6 kWh/kg cell) | [124] |
| C | C. pyrenoidosa | 79.2% @ 1 min (0.3 kWh/kg cell) | [126] | |
| Technique | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Auto-flocculation | Spontaneous aggregation and sedimentation under stress conditions |
|
|
| Bio-flocculation | Co-pelletization of target algal species using bio-flocculants (fungi, bacteria, yeast, algae and their extracellular polymers) |
|
|
| Chemical flocculation | Charge neutralization, bridging, and sweeping of algal cells with charged chemicals |
|
|
| Particle-based flocculation | Charge neutralization and electrostatic bridging with functional nano/macro-particles |
|
|
| Electrochemical flocculation | Floc formation using metal ions and charge neutralization by passing direct electrical current through electrodes |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.-E.; Lee, Y.-C.; Oh, Y.-K. Flocculation Harvesting Techniques for Microalgae: A Review. Appl. Sci. 2019, 9, 3069. https://0-doi-org.brum.beds.ac.uk/10.3390/app9153069
Matter IA, Bui VKH, Jung M, Seo JY, Kim Y-E, Lee Y-C, Oh Y-K. Flocculation Harvesting Techniques for Microalgae: A Review. Applied Sciences. 2019; 9(15):3069. https://0-doi-org.brum.beds.ac.uk/10.3390/app9153069
Chicago/Turabian StyleMatter, Ibrahim A., Vu Khac Hoang Bui, Mikyoung Jung, Jung Yoon Seo, Young-Eun Kim, Young-Chul Lee, and You-Kwan Oh. 2019. "Flocculation Harvesting Techniques for Microalgae: A Review" Applied Sciences 9, no. 15: 3069. https://0-doi-org.brum.beds.ac.uk/10.3390/app9153069