1. Introduction
As a renewable energy source, biomass contributes about 15% to the total global energy consumption. In China, a traditional agricultural country, the use of straw, sugar cane, and rice husk has great potential for future energy and fuels [
1]. It is reported that total agricultural and forestry waste in China reaches 1.5 billion tons per year, of which straw has attracted increasing interests due to environmental concerns as most of it is burned on farmland. If straw were used, the bioenergy from available sources would be equivalent to 180 million tons of standard coal [
2]. If one takes into account the CO
2 uptake by plants during growth and supposes that the biomass is processed efficiently for energy and fuels, CO
2 emissions can be reduced by about 90% compared with fossil energy [
3]. There are various processes for converting biomass to energy and fuels including thermochemical processes and bioprocesses. It is believed that the thermochemical processes, including combustion, gasification pyrolysis, and liquefaction, are most promising in the short and medium terms of 5 to 10 years [
4]. In recent years, extensive studies have been reported on the commercialization of these technologies [
5]. However, most of these studies were conducted at pilot or demonstration scales due to the difficulties and challenges in constructing full commercial-scale plants.
Aspen Plus, a large-scale chemical process simulation software, has been widely used in the design and optimization of unit operation devices such as distillation, absorption, and gas fractionation in chemical processes. Optimizing and improving the production itself using the software’s own analysis tools (sensitivity analysis, design regulations, etc.) can reduce costs for capital and operation, in addition to saving energy and avoiding negative impacts on the environment. Based on the experimental studies carried out on the fast pyrolysis process of biomass, Susanne et al. [
6,
7] carried out a simulation design for the process of hydrogenation of bio-oil to biodiesel. In these studies, the simulation of the whole process from raw biomass materials through pyrolysis, bio-oil hydrogenation to transportation fuel was established. Atsonios et al. [
8] also conducted research on the hydrotreating of pyrolysis oil produced by the co-pyrolysis of coal and biomass. They used specific components of the oil including anisole, guaiacol, acetic acid, ethylene, and furan as model compounds to represent the pyrolysis oils. The effect of the co-pyrolysis, hydrogenation, catalytic reforming hydrogen production, and coke combustion on the overall process was investigated. In a separate study, Wright et al. [
9] simulated the hydrogenation process of bio-oil produced by the rapid pyrolysis of cornstalk, and conducted economic and technical assessments of the entire process. It is found that hydrogen sources have significant impacts which include hydrogen production from reforming part of the pyrolysis bio-oil and hydrogen purchased from the commercial process of natural gas reforming. The results show that if the hydrogen is obtained from reforming pyrolysis bio-oil, the final liquid product yield has the greatest impact on the whole process economy. However, if the hydrogen is purchased from the commercial process, the biomass raw material price has the greatest impact on the entire process economy.
In this study, an Aspen Plus-based process model was established to simulate the two stages of bio-oil hydrogenation. The unique feature of the model was that it considered intrinsic reaction kinetics of catalyst coking deactivation in the first mild stage and the influences of the whole production process. The model also included material mass balance and energy balance in the bio-oil hydrogenation process under different reaction conditions. The process was finally optimized based on economic analysis to achieve the lowest operating costs and the lowest capital costs.
2. Development of a Process Model for Two-Stage Bio-Oil Hydrogenation (HDO)
2.1. Specification of Compounds from Bio-Oil
A process model was developed by using Aspen Plus to simulate the process of two-stage bio-oil hydrogenation. According to the properties of raw bio-oil and products shown in
Table 1, and for simulation purposes, the hundreds of substances presenting bio-oil and products were therefore divided into 9 model components according to the reported study [
10]: (1) BIO-OIL (organic compounds of raw bio-oil, CH
1.47O
0.56); (2) H
2; (3) ODF (oil-phase organic compounds of mild stage, CH
1.47O
0.11); (4) AQO (aqueous phase organic compounds of mild stage, CH
3.02O
1.09); (5) CO
2; (6) H
2O; (7) COKES (soluble coke deposit, Cs); (8) COKEIS (insoluble coke deposit, C
is); and (9) PRODUCT (model compound that represents the final product of bio-fuel, with same properties of gasoline, CH
1.71). Model compounds were selected from the Aspen Plus database to represent the above-mentioned substances in the bio-oil and the upgraded products [
11]. The developed HDO process model is adaptable for different types of bio-oil feedstock with given properties and reaction kinetic parameters.
2.2. Process Setup
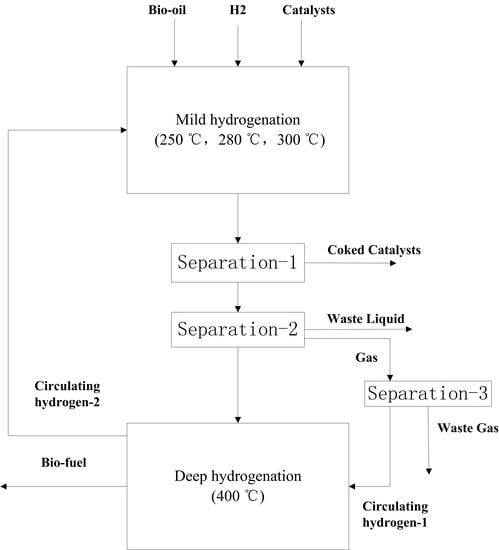
As discussed above, the whole HDO process was divided into two main stages which included a mild hydrogenation stage and a subsequent deep hydrogenation stage. The system flow diagram for the bio-oil hydrogenation process is shown in
Figure 1 and
Figure 2. The whole HDO process consists of seven unit operations: (1) hydrogen preheating; (2) bio-oil preheating; (3) mild hydrogenation reactor; (4) mild hydrogenation product solid–liquid separation; (5) mild hydrogenation product liquid–gas separation; (6) deep hydrogenation reaction; and (7) product cooling and pressure separation.
The organic compounds of raw bio-oil (BIO-OIL), hydrogen (H2-1), and the catalyst (CATALYST) are treated as input parameters under normal conditions that are first preheated by the stream (MIXTURE3) and then continuously fed into the first stage HDO, or the mild hydrogenation reactor (RCSTR). According to the reported research and experimental data, operating temperature for the mild hydrogenation reactor of raw bio-oil is set at 250 °C, 280 °C, and 300 °C, respectively. The kinetic reactions added to this RCSTR reactor include the main bio-oil hydrogenation reaction plus side reactions of the catalyst coking [
12].
The product stream (MIXTURE1) from mild hydrogenation reactor first enters a solid-fluidized bed separator (SEP-1) to remove the used catalysts (with soluble and insoluble coke deposits), and the liquid product then flows into a three-phase separator (SEP-2) to be separated into: (1) water and aqueous phase organic compounds (AQO) formed during the hydrogenation reaction; (2) the unreacted bio-oil with an oil-phase organic compound (ODF); and (3) a mixture of gaseous products and unreacted hydrogen (GAS-1). The organic compound (ODF) and unreacted hydrogen (H2-2) are separated from stream (GAS-1) and then fed into the secondary hydrogenation reactor (RSTOIC) for the deep hydrogenation. The product stream (MIXTURE3) is separated after the heat exchanger. Excessive hydrogen (H2-C) from the deep hydrogenation is continuously recycled and fed to the mild reactor. The heat recovery of the process is achieved by a series of heat exchangers between process streams and feedstock, and the only unit operation that requires external heat supply within the entire process is the secondary hydrogenation reactor.
2.3. Determination of Properties of Bio-Oil and Upgraded Products
The bio-oil and its upgraded product components in this study are complicated, mostly non-polar mixtures of acids, substances, aldehydes, ethers, and alcohols. Therefore, the PR-BM method was chosen in Aspen Plus to determine the chemical and physical properties for both bio-oil and its upgraded products.
2.4. Input Parameters for Plant Operation and Simulation of Hydrogenation Upgrading of Bio-Oil
The annual throughput of the demonstration plant in this case study was set at 107 thousand tons per year of raw bio-oil. Therefore, the input flow rates of feedstock materials were set as follows: 10,000 kg/h for the organic compounds of raw bio-oil (BIO-OIL), 345 kg/h for the hydrogen (H2), and 10 kg/h for the fresh catalyst feed (CATALYST). Catalyst loading in the reactor (RCSTR) was 200 kg. The ratios among the feed streams were selected from reported values [
13]. The Ni-based commercial catalysts were used in the mild stage of hydrogenation (HDO). Three sets of operating conditions for mild HDO step were assessed which were, respectively, (1) 250 °C, 5.6 MPa; (2) 280 °C, 8 MPa; and (3) 300 °C, 10 MPa. The optimum reaction conditions were eventually selected according to the desired product yield.
According to the theory of mild hydrogenation kinetics, the chemical conversion reactions among the components was established [
10]. The model for the conversion of the catalytic bio-oil hydrogenation process was obtained by fitting the experimental data of product distribution and catalyst deactivation in the small industrial reaction [
14]. The pre-exponential factor A and the activation energy E in the chemical reactions were obtained from the previous study on kinetic modeling of the bio-oil hydrogenation from which the parameters of the mild-stage reactions were fitted. Their values are given in
Table 2, with the parameter setting of reaction conditions as described above.
The deactivation rate of the catalyst is defined as follows:
where m
cat is the total mass of the catalyst at a given time and m
cat,f is the mass of fresh catalyst. The relationship between the deactivation rate of the catalyst and catalyst coking has been derived from coking theory [
14] and experimental results of the batch hydrogenation reactor [
15] and is expressed as follows:
where K
d is the deactivation constant of coke deposition, α is the weighing factor of coke type, and β is the deactivation exponent. The mathematical expression of impacts of catalyst coking deactivation (Equation (2)) on the reaction system in the mild HDO stage is embedded into the Aspen model using a FORTRAN subroutine.
The deep hydrogenation step used a Ni/Al2O3 catalyst, under the operating conditions of 400 °C and 15 MPa.
The stoichiometry for deep hydrogenation reaction which produces the final upgraded bio-fuel (PRODUCT) is obtained from reported experimental results [
16] and shown in Equation (3).