Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the As-Prepared GO Composite
2.2.2. Characterization
2.2.3. Adsorption Experiment
3. Results and Discussion
3.1. Characterization
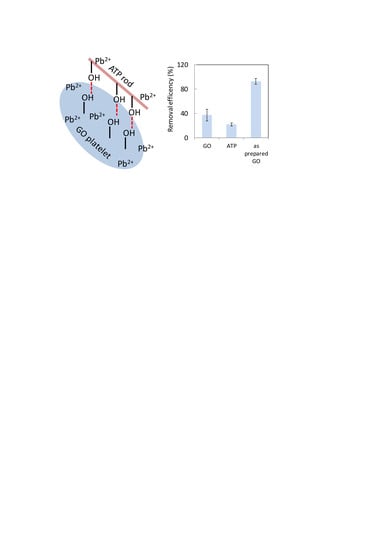
3.2. Comparison of Adsorbents
3.3. Effect of pH
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
3.6. Thermodynamic Parameters
3.7. Regeneration and Recycling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hudcová, B.; Veselská, V.; Filip, J.; Číhalová, S.; Komárek, M. Highly effective Zn(II) and Pb(II) removal from aqueous solutions using Mg-Fe layered double hydroxides: Comprehensive adsorption modeling coupled with solid state analyses. J. Clean. Prod. 2018, 171, 944–953. [Google Scholar] [CrossRef]
- Saeidi, N.; Parvini, M.; Niavarani, Z. High surface area and mesoporous graphene/activated carbon composite for adsorption of Pb(II) from wastewater. J. Environ. Chem. Eng. 2015, 3 Pt A, 2697–2706. [Google Scholar] [CrossRef]
- Thanh, H.T.M.; Phuong, T.T.T.; Hang, P.T.L.; Toan, T.T.T.; Tuyen, T.N.; Mau, T.X.; Khieu, D.Q. Comparative study of Pb(II) adsorption onto MIL–101 and Fe–MIL–101 from aqueous solutions. J. Environ. Chem. Eng. 2018, 6, 4093–4102. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Meunier, N.; Drogui, P.; Montané, C.; Hausler, R.; Mercier, G.; Blais, J.-F. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, M.; Bulut, V.N.; Elvan, H.; Ozdes, D.; Soylak, M.; Duran, C. Determination of Pb(II), Zn(II), Cd(II), and Co(II) ions by flame atomic absorption spectrometry in food and water samples after preconcentration by coprecipitation with Mo(VI)-diethyldithiocarbamate. Environ. Monit. Assess. 2013, 185, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.G.; Shah, F.; Afridi, H.I.; Khan, S.; Arian, S.S.; Brahman, K.D. A green preconcentration method for determination of cobalt and lead in fresh surface and waste water samples prior to flame atomic absorption spectrometry. J. Anal. Methods Chem. 2012, 2012, 713862. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Yuan, D.; Li, Q.; Wu, X. The study of lead removal from aqueous solution using an electrochemical method with a stainless steel net electrode coated with single wall carbon nanotubes. Chem. Eng. J. 2013, 218, 81–88. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; Zeng, G.; Huang, J.; Xu, H.; Zhang, Y.; Wei, S.; Wang, L. The adsorption behavior and mechanism investigation of Pb(II) removal by flocculation using microbial flocculant GA1. Bioresour. Technol. 2013, 148, 414–421. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: Selectivity determination and influence of acidity on metal uptake. J. Colloid Interface Sci. 2003, 261, 49–54. [Google Scholar] [CrossRef]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Heidary, F.; Salehi, E. Co-adsorption/filtration of heavy metal ions from water using regenerated cellulose UF membranes modified with DETA ligand. Sep. Sci. Technol. 2013, 48, 1308–1314. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X.; Chen, C.; Li, G.; Meng, Y. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead(II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, J.-L.; Zenga, G.-M.; Ou, X.-M.; Jiang, Y.; Chang, Y.-N.; Guo, M.; Zhang, C.; Liu, H.-Y. Simultaneous removal of humic acid/fulvic acid and lead from landfill leachate using magnetic graphene oxide. Appl. Surf. Sci. 2016, 370, 335–350. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Li, X.; Qiu, H. Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surf. B Biointerfaces 2013, 103, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, X.; Zhou, G.; Liu, C.; Tang, Y.; Liu, Y. Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb(II) from wastewater. J. Hazard. Mater. 2014, 274, 145–155. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaría, R.; Menéndez, R.; Corma, A.; García, H. Surface area measurement of graphene oxide in aqueous solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, P.; Zhang, L.; Huang, S.; Luo, L.; Huang, C. Toxicity of graphene oxide and multi-walled carbon nanotubes against human cells and zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Gu, D.; Fein, J.B. Adsorption of metals onto graphene oxide: Surface complexation modeling and linear free energy relationships. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 319–327. [Google Scholar] [CrossRef]
- Paredes, J.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, Y.; Mu, B.; Wang, A. Attapulgite/bentonite interactions for methylene blue adsorption characteristics from aqueous solution. Chem. Eng. J. 2014, 237, 403–410. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Tang, Y.; Kou, X. Synthesis of sodium alginate graft poly (acrylic acid-co-2-acrylamido-2-methyl-1-propane sulfonic acid)/attapulgite hydrogel composite and the study of its adsorption. Polym. Plast. Technol. Eng. 2014, 53, 74–79. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wang, A. pH-Responsive nanocomposites from methylcellulose and attapulgite nanorods: Synthesis, swelling and absorption performance on heavy metal ions. J. Macromol. Sci. Part A 2012, 49, 306–315. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Ma, H.; Ji, Y.; Bi, L. Adsorptive removal of humic acid from aqueous solution on polyaniline/attapulgite composite. Chem. Eng. J. 2011, 173, 171–177. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, B. Facile fabrication of crumpled graphene oxide nanosheets and its Platinum nanohybrids for high efficient catalytic activity. Environ. Pollut. 2018, 243, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, S.; Du, W.; Hu, T. Effect of water chemistries on adsorption of Cs(I) onto graphene oxide investigated by batch and modeling techniques. Chem. Eng. J. 2016, 292, 92–97. [Google Scholar] [CrossRef]
- Bradley, W. The structural scheme of attapulgite. Am. Mineral. 1940, 25, 405–410. [Google Scholar]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite superabsorbent composites. Carbohydr. Polym. 2007, 68, 367–374. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Potgieter-Vermaak, S.S.; Kalibantonga, P.D. Heavy metals removal from solution by palygorskite clay. Miner. Eng. 2006, 19, 463–470. [Google Scholar] [CrossRef]
- Wang, S.; Ariyanto, E. Competitive adsorption of malachite green and Pb ions on natural zeolite. J. Colloid Interface Sci. 2007, 314, 25–31. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Ma, B.; Chen, Y. Removal of Pb(II) from aqueous solution using a new zeolite-type absorbent: Potassium ore leaching residue. J. Environ. Chem. Eng. 2018, 6, 7138–7143. [Google Scholar] [CrossRef]
- Liao, B.; Sun, W.-Y.; Guo, N.; Ding, S.-L.; Su, S.-J. Equilibriums and kinetics studies for adsorption of Ni (II) ion on chitosan and its triethylenetetramine derivative. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 32–41. [Google Scholar] [CrossRef]
- Huang, X.; Pan, M. The highly efficient adsorption of Pb (II) on graphene oxides: A process combined by batch experiments and modeling techniques. J. Mol. Liq. 2016, 215, 410–416. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Winzor, D.J. Interpretation of Deviations from Pseudo-First-Order Kinetic Behavior in the Characterization of Ligand Binding by Biosensor Technology. Anal. Biochem. 1996, 236, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon—Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Lanowix, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 30, 1361–1403. [Google Scholar]
- Freundlich, U. Die adsorption in lusungen. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Liu, L.; Li, C.; Bao, C.; Jia, Q.; Xiao, P.; Liu, X.; Zhang, Q. Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 2012, 93, 350–357. [Google Scholar] [CrossRef]
- Niwas, R.; Gupta, U.; Khan, A.A.; Varshney, K.G. The adsorption of phosphamidon on the surface of styrene supported zirconium (IV) tungstophosphate: A thermodynamic study. Colloids Surf. A Physicochem. Eng. Asp. 2000, 164, 115–119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, X.; Liu, M.; Yang, L.; Wu, Z.; Xia, S.; Zhao, J. Adsorption of Pb (II) in aqueous solutions by bamboo charcoal modified with KMnO4 via microwave irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 1–8. [Google Scholar] [CrossRef]
- Li, Y.-H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef] [PubMed]









| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | KF (mg/g) | n | R2 |
| 450.9 | 0.147 | 0.9925 | 116.5 | 3.005 | 0.9127 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, B.; Cheng, X.; Wang, G.; Li, H.; Song, X.; Dai, L. Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution. Appl. Sci. 2019, 9, 1390. https://0-doi-org.brum.beds.ac.uk/10.3390/app9071390
Wei B, Cheng X, Wang G, Li H, Song X, Dai L. Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution. Applied Sciences. 2019; 9(7):1390. https://0-doi-org.brum.beds.ac.uk/10.3390/app9071390
Chicago/Turabian StyleWei, Bigui, Xiabing Cheng, Gang Wang, Hua Li, Xiaosan Song, and Liang Dai. 2019. "Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution" Applied Sciences 9, no. 7: 1390. https://0-doi-org.brum.beds.ac.uk/10.3390/app9071390