Composition and Morphology Characteristics of Magnetic Fractions of Coal Fly Ash Wastes Processed in High-Temperature Exposure in Thermal Power Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Size Analysis
2.2. Characterization
3. Results and Discussion
3.1. The particle Size Distribution
3.2. Mineralogical Analysis
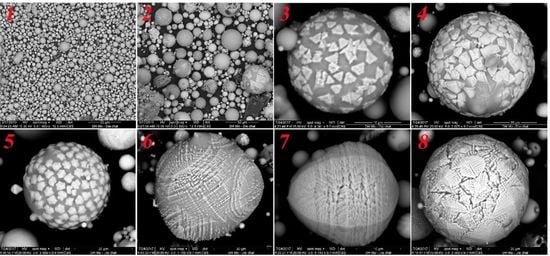
3.3. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y.; Li, N. Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials. Sustainability 2019, 11, 1283. [Google Scholar] [CrossRef]
- Gupta, N.; Gedam, V.V.; Moghe, C.; Labhasetwar, P. Investigation of characteristics and leaching behavior of coal fly ash, coal fly ash bricks and clay bricks. Environ. Technol. Innov. 2017, 7, 152–159. [Google Scholar]
- Carlson, C.L.; Adriano, D.C. Environmental impacts of coal combustion residues. J. Environ. Qual. 1993, 22, 227–247. [Google Scholar] [CrossRef]
- Kalaw, M.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M. Optimizing and characterizing geopolymers from ternary blend of Philippine coal fly ash, coal bottom ash and rice hull ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Hanum, F.; Desfitri, E.; Hayakawa, Y.; Kambara, S. Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash. Minerals 2018, 8, 493. [Google Scholar] [CrossRef]
- Tomeczek, J.; Palugniok, H. Kinetics of mineral matter transformation during coal combustion. Fuel 2002, 81, 1251–1258. [Google Scholar] [CrossRef]
- Grau, F.; Choo, H.; Hu, J.; Jung, J. Engineering behavior and characteristics of wood ash and sugarcane bagasse ash. Materials 2015, 8, 6962–6977. [Google Scholar] [CrossRef]
- Li, X.Y.; Ma, X.W.; Zhang, S.J.; Zheng, E.Z. Mechanical Properties and Microstructure of Class C Fly Ash-Based Geopolymer Paste and Mortar. Materials 2013, 6, 1485–1495. [Google Scholar] [CrossRef] [Green Version]
- Tigue, A.A.S.; Malenab, R.A.J.; Dungca, J.R.; Yu, D.E.C.; Promentilla, M.A.B. Chemical Stability and Leaching Behavior of One-Part Geopolymer from Soil and Coal Fly Ash Mixtures. Minerals 2018, 8. [Google Scholar] [CrossRef]
- Du Plessis, P.W.; Ojumu, T.V.; Fatoba, O.O.; Akinyeye, R.O.; Petrik, L.F. Distributional Fate of Elements during the Synthesis of Zeolites from South African Coal Fly Ash. Materials 2014, 7, 3305–3318. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, P.W.; Ojumu, T.V.; Petrik, L.F. Waste Minimization Protocols for the Process of Synthesizing Zeolites from South African Coal Fly Ash. Materials 2013, 6, 1688–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darsanasiri, A.; Matalkah, F.; Ramli, S.; Al-Jalode, K.; Balachandra, A.; Soroushian, P. Ternary alkali aluminosilicate cement based on rice husk ash, slag and coal fly ash. J. Build. Eng. 2018, 19, 36–41. [Google Scholar] [CrossRef]
- Mashau, A.S.; Gitari, M.W.; Akinyemi, S.A. Evaluation of the Bioavailability and Translocation of Selected Heavy Metals by Brassica juncea and Spinacea oleracea L for a South African Power Utility Coal Fly Ash. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Asl, S.M.H.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Clean. Prod. 2019, 208, 1131–1147. [Google Scholar]
- Sun, P.; Sun, D.S.; Wang, A.G.; Ge, W.B.; Liu, X.L. Preparation and Properties of Sintered Brick Based on Coal Gangue and Fly Ash. Asian J. Chem. 2014, 26, 1517–1520. [Google Scholar] [CrossRef]
- Van der Merwe, E.M.; Prinsloo, L.C.; Mathebula, C.L.; Swart, H.C.; Coetsee, E.; Doucet, F.J. Surface and bulk characterization of an ultrafine South African coal fly ash with reference to polymer applications. Appl. Surf. Sci. 2014, 317, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Goyal, D. Mineralogical comparison of coal fly ash with soil for use in agriculture. J. Mater. Cycles Waste Manag. 2016, 18, 186–200. [Google Scholar] [CrossRef]
- Kostova, I. Geochemical Characterization and Mercury Content of Feed Coals And Fly Ashes From Russe Thermoelectric Power Plant, Bulgaria. Comptes Rendus De L Academie Bulgare Des Sciences 2017, 70, 829–838. [Google Scholar]
- Wei, C.D.; Cheng, S.; Zhu, F.J.; Tan, X.L.; Li, W.Q.; Zhang, P.P.; Miao, S.D. Digesting high-aluminum coal fly ash with concentrated sulfuric acid at high temperatures. Hydrometallurgy 2018, 180, 41–48. [Google Scholar] [CrossRef]
- Wu, D.Y.; Sul, Y.; Chen, X.C.; He, S.B.; Wang, X.; Kong, H. Changes of mineralogical-chemical composition, cation exchange capacity, and phosphate immobilization capacity during the hydrothermal conversion process of coal fly ash into zeolite. Fuel 2008, 87, 2194–2200. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhang, J.Y.; Tian, C.; Li, H.L.; Shao, X.Y.; Zheng, C.G. Mineralogy and Chemical Composition of High-Calcium Fly Ashes and Density Fractions from a Coal-Fired Power Plant in China. Energy Fuels 2010, 24, 834–843. [Google Scholar]
- Akinyemi, S.A.; Akinlua, A.; Gitari, W.M.; Petrik, L.F. Mineralogy and Mobility Patterns of Chemical Species in Weathered Coal Fly Ash. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 768–784. [Google Scholar] [CrossRef]
- Cvetkovic, Z.; Logar, M.; Rosic, A.; Ciric, A. Mineral composition of the airborne particles in the coal dust and fly ash of the Kolubara basin (Serbia). Periodico Di Mineralogia 2012, 81, 205–223. [Google Scholar]
- Rivera, N.; Kaur, N.; Hesterberg, D.; Ward, C.R.; Austin, R.E.; Duckworth, O.W. Chemical Composition, Speciation, and Elemental Associations in Coal Fly Ash Samples Related to the Kingston Ash Spill. Energy Fuels 2015, 29, 954–967. [Google Scholar]
- Koukouzas, N.; Ward, C.R.; Papanikolaou, D.; Li, Z.S.; Ketikidis, C. Quantitative evaluation of minerals in fly ashes of biomass, coal and biomass-coal mixture derived from circulating fluidised bed combustion technology. J. Hazard. Mater. 2009, 169, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Koukouzas, N.; Ketikidis, C.; Itskos, G. Heavy metal characterization of CFB-derived coal fly ash. Fuel Process. Technol. 2011, 92, 441–446. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Calabrese, D.; Valenti, G.L.; Montagnaro, F. Flue gas desulfurization gypsum and coal fly ash as basic components of prefabricated building materials. Waste Manag. 2013, 33, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Koukouzas, N.K.; Zeng, R.S.; Perdikatsis, V.; Xu, W.D.; Kakaras, E.K. Mineralogy and geochemistry of Greek and Chinese coal fly ash. Fuel 2006, 85, 2301–2309. [Google Scholar] [CrossRef]
- Koukouzas, N.; Hamalainen, J.; Papanikolaou, D.; Tourunen, A.; Jantti, T. Mineralogical and elemental composition of fly ash from pilot scale fluidised bed combustion of lignite, bituminous coal, wood chips and their blends. Fuel 2007, 86, 2186–2193. [Google Scholar] [CrossRef]
- Shoumkova, A.; Stoyanova, V. Zeolites formation by hydrothermal alkali activation of coal fly ash from thermal power station “Maritsa 3’’, Bulgaria. Fuel 2013, 103, 533–541. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Koteja, A.; Franus, W. Influence of the reaction time on the crystal structure of Na-P1 zeolite obtained from coal fly ash microspheres. Microporous Mesoporous Mater. 2018, 266, 102–108. [Google Scholar] [CrossRef]
- He, P.; Jiang, X.M.; Wu, J.; Pan, W.G.; Ren, J.X. Characterization Of Fly Ash From Coal-Fired Power Plant And Their Properties Of Mercury Retention. Surf. Rev. Lett. 2015, 22. [Google Scholar] [CrossRef]
- Izidoro, J.D.; Fungaro, D.A.; dos Santos, F.S.; Wang, S.B. Characteristics of Brazilian coal fly ashes and their synthesized zeolites. Fuel Process. Technol. 2012, 97, 38–44. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andres, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicova, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Shoumkova, A.S. Magnetic separation of coal fly ash from Bulgarian power plants. Waste Manag. Res. 2011, 29, 1078–1089. [Google Scholar]
- Sow, M.; Hot, J.; Tribout, C.; Cyr, M. Characterization of Spreader Stoker Coal Fly Ashes (SSCFA) for their use in cement-based applications. Fuel 2015, 162, 224–233. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Udaybhanu, G.; Basu, A. A comprehensive characterisation of fly ash from a thermal power plant in Eastern India. Fuel Process. Technol. 2006, 87, 259–277. [Google Scholar] [CrossRef]
- Dos Santos, R.P.; Martins, J.; Gadelha, C.; Cavada, B.; Albertini, A.V.; Arruda, F.; Vasconcelos, M.; Teixeira, E.; Alves, F.; Lima, J.; Freire, V. Coal Fly Ash Ceramics: Preparation, Characterization, and Use in the Hydrolysis of Sucrose. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Sokol, E.; Kalugin, V.; Nigmatulina, E.; Volkova, N.; Frenkel, A.; Maksimova, N. Ferrospheres from fly ashes of Chelyabinsk coals: Chemical composition, morphology and formation conditions. Fuel 2002, 81, 867–876. [Google Scholar] [CrossRef]
- Bourliva, A.; Papadopoulou, L.; Aidona, E.; Simeonidis, K.; Vourlias, G.; Devlin, E.; Sanakis, Y. Enrichment and oral bioaccessibility of selected trace elements in fly ash-derived magnetic components. Environ. Sci. Pollut. Res. 2017, 24, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.H.; Zhang, D.X.; Zhang, K.; Cao, Y. Capture of Gas-Phase Arsenic by Ferrospheres Separated from Fly Ashes. Energy Fuels 2016, 30, 8746–8752. [Google Scholar]
- Anshits, N.N.; Fedorchak, M.A.; Zhizhaev, A.M.; Sharonova, O.M.; Anshits, A.G. Composition and Structure of Block-Type Ferrospheres Isolated from Calcium-Rich Power Plant Ash. Inorg. Mater. 2018, 54, 187–194. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Zhizhaev, A.M.; Anshits, A.G. Structure-Composition Relationship of Skeletal and Dendritic Ferrospheres Isolated from Calcium-Rich Power Plant Ash. Inorg. Mater. 2018, 54, 253–260. [Google Scholar] [CrossRef]






| Element (%) | The Concentration of Major Elements (%) of Magnetic Fly Ash | |
|---|---|---|
| UB | PL | |
| SiO2 | 43.64 | 42.85 |
| TiO2 | 0.83 | 0.76 |
| Al2O3 | 21.97 | 21.05 |
| T-Fe2O3 | 23.54 | 22.79 |
| MnO | 0.26 | 0.22 |
| MgO | 1.89 | 1.96 |
| CaO | 1.85 | 1.53 |
| Na2O | 0.03 | 0.05 |
| K2O | 2.47 | 2.71 |
| P2O5 | 0.22 | 0.2 |
| SO3 | 0.34 | 0.47 |
| LOI * | 3.0 | 5.6 |
| Size (µm) | Content (%) | |||||
|---|---|---|---|---|---|---|
| Uong Bi (UB) Fly Ash | Pha Lai (PL) Fly Ash | |||||
| Fly ash | Magnetic | Nonmagnetic | Fly ash | Magnetic | Nonmagnetic | |
| >250 | 0.41 | 0.14 | 0.27 | 0.33 | 0.12 | 0.21 |
| 90–250 | 16.48 | 2.03 | 14.45 | 14.48 | 2.59 | 11.89 |
| 45–90 | 25.58 | 3.52 | 22.06 | 31.19 | 4.62 | 26.57 |
| 32–45 | 31.18 | 2.43 | 28.75 | 36.66 | 3.23 | 33.43 |
| <32 | 26.24 | 2.11 | 24.13 | 16.93 | 2.51 | 14.42 |
| Total | 99.89 | 10.23 | 89.66 | 99.59 | 13.07 | 86.52 |
| Element (%) | The Concentration of Trace Elements (ppm) of Magnetic Fly Ash | |
|---|---|---|
| UB | PL | |
| Co | 26.13 | 34.27 |
| Ni | - | - |
| Cu | 42.92 | 39.38 |
| Zn | 15.51 | - |
| Mo | 1.24 | 1.15 |
| Cd | - | 0.23 |
| Pb | 27.22 | 21.73 |
| Mn | 739.38 | 1237.46 |
| Mg | 700.94 | 754.2 |
| Cr | 148.75 | 132.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, D.-H.; Bui, H.-B.; Kalantar, B.; Bui, X.-N.; Nguyen, D.-A.; Le, Q.-T.; Do, N.-H.; Nguyen, H. Composition and Morphology Characteristics of Magnetic Fractions of Coal Fly Ash Wastes Processed in High-Temperature Exposure in Thermal Power Plants. Appl. Sci. 2019, 9, 1964. https://0-doi-org.brum.beds.ac.uk/10.3390/app9091964
Vu D-H, Bui H-B, Kalantar B, Bui X-N, Nguyen D-A, Le Q-T, Do N-H, Nguyen H. Composition and Morphology Characteristics of Magnetic Fractions of Coal Fly Ash Wastes Processed in High-Temperature Exposure in Thermal Power Plants. Applied Sciences. 2019; 9(9):1964. https://0-doi-org.brum.beds.ac.uk/10.3390/app9091964
Chicago/Turabian StyleVu, Dinh-Hieu, Hoang-Bac Bui, Bahareh Kalantar, Xuan-Nam Bui, Dinh-An Nguyen, Qui-Thao Le, Ngoc-Hoan Do, and Hoang Nguyen. 2019. "Composition and Morphology Characteristics of Magnetic Fractions of Coal Fly Ash Wastes Processed in High-Temperature Exposure in Thermal Power Plants" Applied Sciences 9, no. 9: 1964. https://0-doi-org.brum.beds.ac.uk/10.3390/app9091964