Molecular Interventions towards Multiple Sclerosis Treatment
Abstract
:1. Introduction
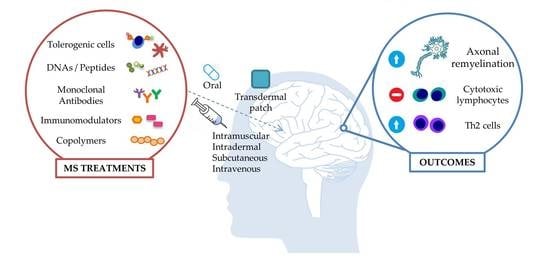
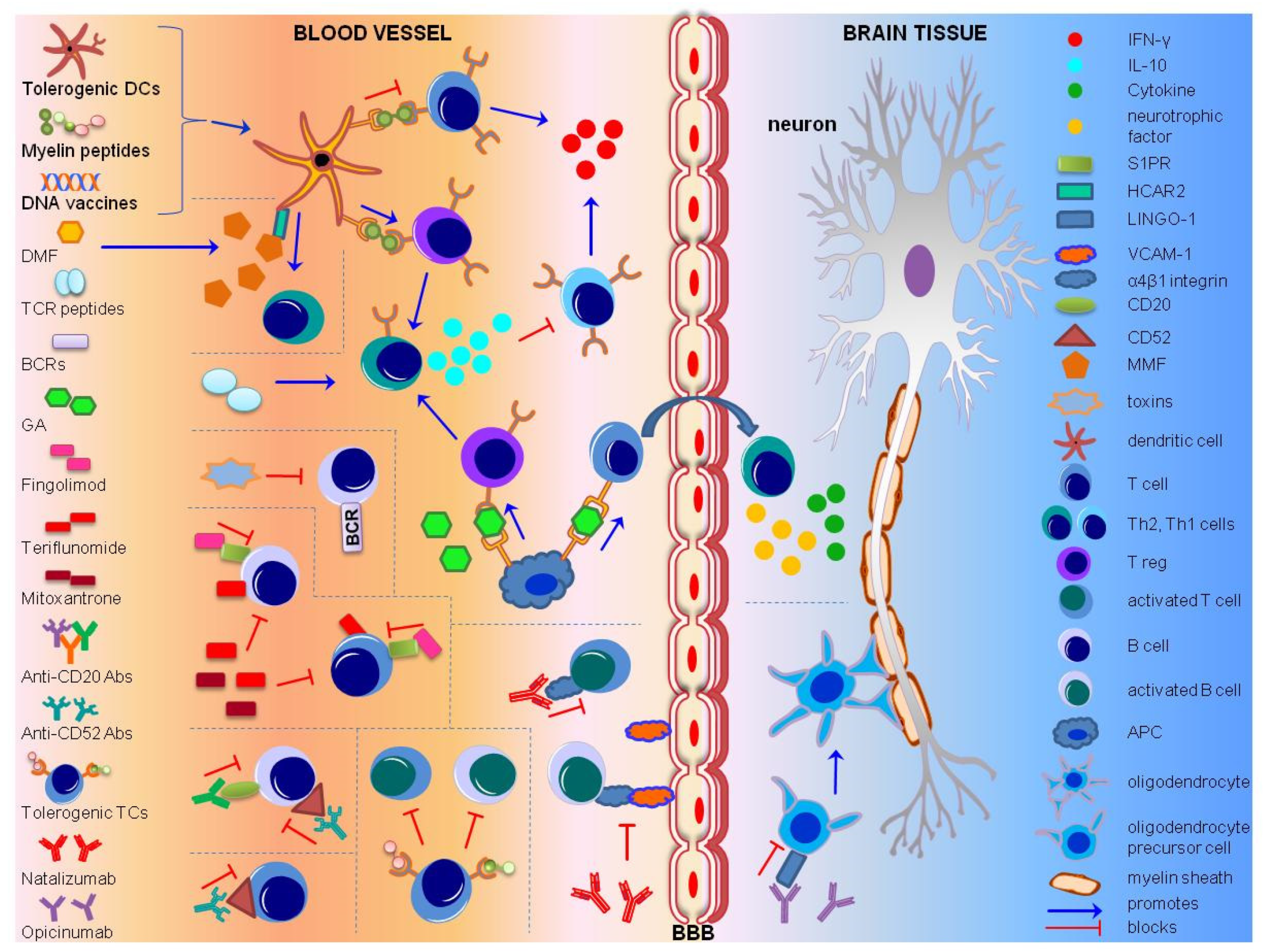
2. Immunotherapeutic Approaches
2.1. Antigen-Specific Immunotherapy (ASI)
2.2. Cell-specific Immunotherapy
2.3. Cell Receptor-Specific Immunotherapy
2.4. Monoclonal Antibodies (MABs)
2.5. HLA Antagonistic Co-polymers
3. Delivery Methods of Immunotherapeutic Factors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Grigoriadis, N.; van Pesch, V. A basic overview of multiple sclerosis immunopathology. Eur. J. Neurol. 2015, 22 (Suppl. 2), 3–13. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sorensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet. Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Epidemiology of multiple sclerosis. Does this really point toward an etiology? Lectio doctoralis. Neurol. Sci. 2000, 21, 383–403. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin d and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Oksenberg, J.R. Decoding multiple sclerosis: An update on genomics and future directions. Expert Rev. Neurother. 2013, 13, 11–19. [Google Scholar] [CrossRef]
- Ascherio, A. Environmental factors in multiple sclerosis. Expert Rev. Neurother. 2013, 13, 3–9. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Dobson, R.; Meier, U.C.; Giovannoni, G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet. Neurol. 2010, 9, 727–739. [Google Scholar] [CrossRef]
- Hedstrom, A.K.; Baarnhielm, M.; Olsson, T.; Alfredsson, L. Tobacco smoking, but not swedish snuff use, increases the risk of multiple sclerosis. Neurology 2009, 73, 696–701. [Google Scholar] [CrossRef]
- Bjartmar, C.; Wujek, J.R.; Trapp, B.D. Axonal loss in the pathology of ms: Consequences for understanding the progressive phase of the disease. J. Neurol. Sci. 2003, 206, 165–171. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [Green Version]
- de Sa, J.C.; Airas, L.; Bartholome, E.; Grigoriadis, N.; Mattle, H.; Oreja-Guevara, C.; O’Riordan, J.; Sellebjerg, F.; Stankoff, B.; Vass, K.; et al. Symptomatic therapy in multiple sclerosis: A review for a multimodal approach in clinical practice. Ther. Adv. Neurol. Disord. 2011, 4, 139–168. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, R.M.; Hafler, D.A.; Lucchinetti, C.F. Multiple sclerosis-a quiet revolution. Nat. Rev. Neurol. 2015, 11, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Critchfield, J.M.; Racke, M.K.; Zuniga-Pflucker, J.C.; Cannella, B.; Raine, C.S.; Goverman, J.; Lenardo, M.J. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science 1994, 263, 1139–1143. [Google Scholar] [CrossRef]
- Shakya, A.K.; Nandakumar, K.S. Antigen-specific tolerization and targeted delivery as therapeutic strategies for autoimmune diseases. Trends Biotechnol. 2018, 36, 686–699. [Google Scholar] [CrossRef]
- Willekens, B.; Cools, N. Beyond the magic bullet: Current progress of therapeutic vaccination in multiple sclerosis. CNS Drugs 2018, 32, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Bielekova, B.; Goodwin, B.; Richert, N.; Cortese, I.; Kondo, T.; Afshar, G.; Gran, B.; Eaton, J.; Antel, J.; Frank, J.A.; et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase ii clinical trial with an altered peptide ligand. Nat. Med. 2000, 6, 1167–1175. [Google Scholar] [CrossRef]
- Schrempf, W.; Ziemssen, T. Glatiramer acetate: Mechanisms of action in multiple sclerosis. Autoimmun. Rev. 2007, 6, 469–475. [Google Scholar] [CrossRef]
- Noon, L. Prophylactic inoculation against hay fever. Int. Arch. Allergy Appl. Immunol. 1953, 4, 285–288. [Google Scholar] [CrossRef] [Green Version]
- Frankland, A.W.; Augustin, R. Prophylaxis of summer hay-fever and asthma: A controlled trial comparing crude grass-pollen extracts with the isolated main protein component. Lancet 1954, 266, 1055–1057. [Google Scholar] [CrossRef]
- Freeman, J. “ Rush “ inoculation, with special reference to hay-fever treatment. Lancet 1930, 215, 744–747. [Google Scholar] [CrossRef]
- Hochfelder, J.L.; Ponda, P. Allergen immunotherapy: Routes, safety, efficacy, and mode of action. Immunotargets Ther. 2013, 2, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Pozsgay, J.; Szekanecz, Z.; Sarmay, G. Antigen-specific immunotherapies in rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 525–537. [Google Scholar] [CrossRef]
- Hohlfeld, R.; Wekerle, H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: From pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. 2), 14599–14606. [Google Scholar] [CrossRef] [Green Version]
- Karin, N.; Mitchell, D.J.; Brocke, S.; Ling, N.; Steinman, L. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J. Exp. Med. 1994, 180, 2227–2237. [Google Scholar] [CrossRef] [Green Version]
- Stepanov, A.; Lomakin, Y.; Gabibov, A.; Belogurov, A. Peptides against autoimmune neurodegeneration. Curr. Med. Chem. 2017, 24, 1761–1771. [Google Scholar] [CrossRef]
- Puentes, F.; Dickhaut, K.; Hofstatter, M.; Falk, K.; Rotzschke, O. Active suppression induced by repetitive self-epitopes protects against eae development. PLoS ONE 2013, 8, e64888. [Google Scholar] [CrossRef]
- Metzler, B.; Wraith, D.C. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: Influence of mhc binding affinity. Int. Immunol. 1993, 5, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Tselios, T.; Aggelidakis, M.; Tapeinou, A.; Tseveleki, V.; Kanistras, I.; Gatos, D.; Matsoukas, J. Rational design and synthesis of altered peptide ligands based on human myelin oligodendrocyte glycoprotein 35–55 epitope: Inhibition of chronic experimental autoimmune encephalomyelitis in mice. Molecules 2014, 19, 17968–17984. [Google Scholar] [CrossRef] [PubMed]
- Yeste, A.; Nadeau, M.; Burns, E.J.; Weiner, H.L.; Quintana, F.J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2012, 109, 11270–11275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, L.B.; Greer, J.M.; Sobel, R.A.; Lees, M.B.; Kuchroo, V.K. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity 1995, 3, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Kuchroo, V.K.; Greer, J.M.; Kaul, D.; Ishioka, G.; Franco, A.; Sette, A.; Sobel, R.A.; Lees, M.B. A single tcr antagonist peptide inhibits experimental allergic encephalomyelitis mediated by a diverse t cell repertoire. J. Immunol. 1994, 153, 3326–3336. [Google Scholar]
- Walczak, A.; Siger, M.; Ciach, A.; Szczepanik, M.; Selmaj, K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol. 2013, 70, 1105–1109. [Google Scholar] [CrossRef]
- Jurynczyk, M.; Walczak, A.; Jurewicz, A.; Jesionek-Kupnicka, D.; Szczepanik, M.; Selmaj, K. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann. Neurol. 2010, 68, 593–601. [Google Scholar] [CrossRef]
- Chataway, J.; Martin, K.; Barrell, K.; Sharrack, B.; Stolt, P.; Wraith, D.C. Effects of atx-ms-1467 immunotherapy over 16 weeks in relapsing multiple sclerosis. Neurology 2018, 90, e955–e962. [Google Scholar] [CrossRef]
- Crowe, P.D.; Qin, Y.; Conlon, P.J.; Antel, J.P. Nbi-5788, an altered mbp83–99 peptide, induces a t-helper 2-like immune response in multiple sclerosis patients. Ann. Neurol. 2000, 48, 758–765. [Google Scholar] [CrossRef]
- Lomakin, Y.; Belogurov, A., Jr.; Glagoleva, I.; Stepanov, A.; Zakharov, K.; Okunola, J.; Smirnov, I.; Genkin, D.; Gabibov, A. Administration of myelin basic protein peptides encapsulated in mannosylated liposomes normalizes level of serum tnf-alpha and il-2 and chemoattractants ccl2 and ccl4 in multiple sclerosis patients. Mediat. Inflamm. 2016, 2016, 2847232. [Google Scholar] [CrossRef] [Green Version]
- Lutterotti, A.; Yousef, S.; Sputtek, A.; Sturner, K.H.; Stellmann, J.P.; Breiden, P.; Reinhardt, S.; Schulze, C.; Bester, M.; Heesen, C.; et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci. Transl. Med. 2013, 5, 188ra175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappos, L.; Comi, G.; Panitch, H.; Oger, J.; Antel, J.; Conlon, P.; Steinman, L. Induction of a non-encephalitogenic type 2 t helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase ii trial. The altered peptide ligand in relapsing ms study group. Nat. Med. 2000, 6, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Eagar, T.N.; Strominger, J.L.; Miller, S.D. Differential induction of ige-mediated anaphylaxis after soluble vs. Cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2005, 102, 9595–9600. [Google Scholar] [CrossRef] [Green Version]
- Warren, K.G.; Catz, I.; Wucherpfennig, K.W. Tolerance induction to myelin basic protein by intravenous synthetic peptides containing epitope p85 vvhffknivtp96 in chronic progressive multiple sclerosis. J. Neurol. Sci. 1997, 152, 31–38. [Google Scholar] [CrossRef]
- Weiner, H.L.; Mackin, G.A.; Matsui, M.; Orav, E.J.; Khoury, S.J.; Dawson, D.M.; Hafler, D.A. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science 1993, 259, 1321–1324. [Google Scholar] [CrossRef]
- Fissolo, N.; Montalban, X.; Comabella, M. DNA-based vaccines for multiple sclerosis: Current status and future directions. Clin. Immunol. 2012, 142, 76–83. [Google Scholar] [CrossRef]
- Stuve, O.; Cravens, P.D.; Eagar, T.N. DNA-based vaccines: The future of multiple sclerosis therapy? Expert Rev. Neurother. 2008, 8, 351–360. [Google Scholar] [CrossRef]
- Garren, H.; Robinson, W.H.; Krasulova, E.; Havrdova, E.; Nadj, C.; Selmaj, K.; Losy, J.; Nadj, I.; Radue, E.W.; Kidd, B.A.; et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann. Neurol. 2008, 63, 611–620. [Google Scholar] [CrossRef]
- Bar-Or, A.; Vollmer, T.; Antel, J.; Arnold, D.L.; Bodner, C.A.; Campagnolo, D.; Gianettoni, J.; Jalili, F.; Kachuck, N.; Lapierre, Y.; et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch. Neurol. 2007, 64, 1407–1415. [Google Scholar] [CrossRef] [Green Version]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef]
- Zhang, J.; Raus, J. T cell vaccination in multiple sclerosis: Hopes and facts. Acta Neurol. Belg. 1994, 94, 112–115. [Google Scholar]
- Medaer, R.; Stinissen, P.; Truyen, L.; Raus, J.; Zhang, J. Depletion of myelin-basic-protein autoreactive t cells by t-cell vaccination: Pilot trial in multiple sclerosis. Lancet 1995, 346, 807–808. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Rivera, V.M.; Tejada-Simon, M.V.; Yang, D.; Hong, J.; Li, S.; Haykal, H.; Killian, J.; Zang, Y.C. T cell vaccination in multiple sclerosis: Results of a preliminary study. J. Neurol. 2002, 249, 212–218. [Google Scholar] [CrossRef]
- Loftus, B.; Newsom, B.; Montgomery, M.; Von Gynz-Rekowski, K.; Riser, M.; Inman, S.; Garces, P.; Rill, D.; Zhang, J.; Williams, J.C. Autologous attenuated t-cell vaccine (tovaxin) dose escalation in multiple sclerosis relapsing-remitting and secondary progressive patients nonresponsive to approved immunomodulatory therapies. Clin. Immunol. 2009, 131, 202–215. [Google Scholar] [CrossRef]
- Achiron, A.; Lavie, G.; Kishner, I.; Stern, Y.; Sarova-Pinhas, I.; Ben-Aharon, T.; Barak, Y.; Raz, H.; Lavie, M.; Barliya, T.; et al. T cell vaccination in multiple sclerosis relapsing-remitting nonresponders patients. Clin. Immunol. 2004, 113, 155–160. [Google Scholar] [CrossRef]
- Karussis, D.; Shor, H.; Yachnin, J.; Lanxner, N.; Amiel, M.; Baruch, K.; Keren-Zur, Y.; Haviv, O.; Filippi, M.; Petrou, P.; et al. T cell vaccination benefits relapsing progressive multiple sclerosis patients: A randomized, double-blind clinical trial. PLoS ONE 2012, 7, e50478. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Raker, V.K.; Domogalla, M.P.; Steinbrink, K. Tolerogenic dendritic cells for regulatory t cell induction in man. Front. Immunol. 2015, 6, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.P.; Willekens, B.; Cras, P.; Goossens, H.; Martinez-Caceres, E.; Berneman, Z.N.; Cools, N. Immunomodulatory effects of 1,25-dihydroxyvitamin d3 on dendritic cells promote induction of t cell hyporesponsiveness to myelin-derived antigens. J. Immunol. Res. 2016, 2016, 5392623. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Selles-Moreno, C.; Fabregas-Puig, S.; Amoedo, J.; Navarro-Barriuso, J.; Teniente-Serra, A.; Grau-Lopez, L.; Ramo-Tello, C.; Martinez-Caceres, E.M. Beneficial effect of tolerogenic dendritic cells pulsed with mog autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2015, 21, 222–230. [Google Scholar] [CrossRef]
- Zubizarreta, I.; Florez-Grau, G.; Vila, G.; Cabezon, R.; Espana, C.; Andorra, M.; Saiz, A.; Llufriu, S.; Sepulveda, M.; Sola-Valls, N.; et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc. Natl. Acad. Sci. USA 2019, 116, 8463–8470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewell, A.K. Why must t cells be cross-reactive? Nat. Rev. Immunol. 2012, 12, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Vandenbark, A.A.; Hashim, G.; Offner, H. Immunization with a synthetic t-cell receptor v-region peptide protects against experimental autoimmune encephalomyelitis. Nature 1989, 341, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Offner, H.; Hashim, G.A.; Vandenbark, A.A. T cell receptor peptide therapy triggers autoregulation of experimental encephalomyelitis. Science 1991, 251, 430–432. [Google Scholar] [CrossRef]
- Bourdette, D.N.; Whitham, R.H.; Chou, Y.K.; Morrison, W.J.; Atherton, J.; Kenny, C.; Liefeld, D.; Hashim, G.A.; Offner, H.; Vandenbark, A.A. Immunity to tcr peptides in multiple sclerosis. I. Successful immunization of patients with synthetic v beta 5.2 and v beta 6.1 cdr2 peptides. J. Immunol. 1994, 152, 2510–2519. [Google Scholar]
- Vandenbark, A.A.; Chou, Y.K.; Whitham, R.; Mass, M.; Buenafe, A.; Liefeld, D.; Kavanagh, D.; Cooper, S.; Hashim, G.A.; Offner, H. Treatment of multiple sclerosis with t-cell receptor peptides: Results of a double-blind pilot trial. Nat. Med. 1996, 2, 1109–1115. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Culbertson, N.E.; Bartholomew, R.M.; Huan, J.; Agotsch, M.; LaTocha, D.; Yadav, V.; Mass, M.; Whitham, R.; Lovera, J.; et al. Therapeutic vaccination with a trivalent t-cell receptor (tcr) peptide vaccine restores deficient foxp3 expression and tcr recognition in subjects with multiple sclerosis. Immunology 2008, 123, 66–78. [Google Scholar] [CrossRef]
- Gabibov, A.G.; Belogurov, A.A., Jr.; Lomakin, Y.A.; Zakharova, M.Y.; Avakyan, M.E.; Dubrovskaya, V.V.; Smirnov, I.V.; Ivanov, A.S.; Molnar, A.A.; Gurtsevitch, V.E.; et al. Combinatorial antibody library from multiple sclerosis patients reveals antibodies that cross-react with myelin basic protein and ebv antigen. FASEB J. 2011, 25, 4211–4221. [Google Scholar] [CrossRef]
- Lambracht-Washington, D.; O’Connor, K.C.; Cameron, E.M.; Jowdry, A.; Ward, E.S.; Frohman, E.; Racke, M.K.; Monson, N.L. Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid b cells from patients with relapsing remitting ms. J. Neuroimmunol. 2007, 186, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Gilden, D.H.; Ritchie, A.M.; Burgoon, M.P.; Keays, K.M.; Owens, G.P. Specificity of recombinant antibodies generated from multiple sclerosis cerebrospinal fluid probed with a random peptide library. J. Neuroimmunol. 2006, 172, 121–131. [Google Scholar] [CrossRef]
- Stepanov, A.V.; Belogurov, A.A., Jr.; Ponomarenko, N.A.; Stremovskiy, O.A.; Kozlov, L.V.; Bichucher, A.M.; Dmitriev, S.E.; Smirnov, I.V.; Shamborant, O.G.; Balabashin, D.S.; et al. Design of targeted b cell killing agents. PLoS ONE 2011, 6, e20991. [Google Scholar] [CrossRef]
- Madhumathi, J.; Verma, R.S. Therapeutic targets and recent advances in protein immunotoxins. Curr. Opin. Microbiol. 2012, 15, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Voge, N.V.; Alvarez, E. Monoclonal antibodies in multiple sclerosis: Present and future. Biomedicines 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Wootla, B.; Watzlawik, J.O.; Stavropoulos, N.; Wittenberg, N.J.; Dasari, H.; Abdelrahim, M.A.; Henley, J.R.; Oh, S.H.; Warrington, A.E.; Rodriguez, M. Recent advances in monoclonal antibody therapies for multiple sclerosis. Expert Opin. Biol. Ther. 2016, 16, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Yaldizli, O.; Putzki, N. Natalizumab in the treatment of multiple sclerosis. Ther. Adv. Neurol. Disord. 2009, 2, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. New Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. New Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. New Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Lisby, S.; Grove, R.; Derosier, F.; Shackelford, S.; Havrdova, E.; Drulovic, J.; Filippi, M. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: A phase 2 study. Neurology 2014, 82, 573–581. [Google Scholar] [CrossRef]
- Mi, S.; Miller, R.H.; Lee, X.; Scott, M.L.; Shulag-Morskaya, S.; Shao, Z.; Chang, J.; Thill, G.; Levesque, M.; Zhang, M.; et al. Lingo-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 2005, 8, 745–751. [Google Scholar] [CrossRef]
- Mellion, M.; Edwards, K.R.; Hupperts, R.; Drulović, J.; Montalban, X.; Hartung, H.P.; Brochet, B.; Calabresi, P.A.; Rudick, R.; Ibrahim, A.; et al. Efficacy results from the phase 2b synergy study: Treatment of disabling multiple sclerosis with the anti-lingo-1 monoclonal antibody opicinumab (s33.004). Neurology 2017, 88 (Suppl. 16). [Google Scholar]
- Havrdova, E.; Horakova, D.; Kovarova, I. Alemtuzumab in the treatment of multiple sclerosis: Key clinical trial results and considerations for use. Ther. Adv. Neurol. Disord. 2015, 8, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, C.; Goodman, A.D.; Johnson, K.; Kachuck, N.; Lindsey, J.W.; Lisak, R.; Luzzio, C.; Myers, L.; Panitch, H.; Preiningerova, J.; et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: Results from the 15-year analysis of the us prospective open-label study of glatiramer acetate. Mult. Scler. 2010, 16, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P.; Brooks, B.R.; Cohen, J.A.; Ford, C.C.; Goldstein, J.; Lisak, R.P.; Myers, L.W.; Panitch, H.S.; Rose, J.W.; Schiffer, R.B. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase iii multicenter, double-blind placebo-controlled trial. The copolymer 1 multiple sclerosis study group. Neurology 1995, 45, 1268–1276. [Google Scholar] [CrossRef]
- Arnon, R.; Teitelbaum, D.; Sela, M. Suppression of experimental allergic encephalomyelitis by cop1--relevance to multiple sclerosis. Isr. J. Med Sci. 1989, 25, 686–689. [Google Scholar]
- Racke, M.K.; Lovett-Racke, A.E. Glatiramer acetate treatment of multiple sclerosis: An immunological perspective. J. Immunol. 2011, 186, 1887–1890. [Google Scholar] [CrossRef]
- Ponomarenko, N.A.; Durova, O.M.; Vorobiev, I.I.; Belogurov, A.A., Jr.; Kurkova, I.N.; Petrenko, A.G.; Telegin, G.B.; Suchkov, S.V.; Kiselev, S.L.; Lagarkova, M.A.; et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Fridkis-Hareli, M.; Santambrogio, L.; Stern, J.N.; Fugger, L.; Brosnan, C.; Strominger, J.L. Novel synthetic amino acid copolymers that inhibit autoantigen-specific t cell responses and suppress experimental autoimmune encephalomyelitis. J. Clin. Investig. 2002, 109, 1635–1643. [Google Scholar] [CrossRef]
- Salvioni, L.; Fiandra, L.; Del Curto, M.D.; Mazzucchelli, S.; Allevi, R.; Truffi, M.; Sorrentino, L.; Santini, B.; Cerea, M.; Palugan, L.; et al. Oral delivery of insulin via polyethylene imine-based nanoparticles for colonic release allows glycemic control in diabetic rats. Pharmacol. Res. 2016, 110, 122–130. [Google Scholar] [CrossRef]
- Sajeesh, S.; Vauthier, C.; Gueutin, C.; Ponchel, G.; Sharma, C.P. Thiol functionalized polymethacrylic acid-based hydrogel microparticles for oral insulin delivery. Acta Biomater. 2010, 6, 3072–3080. [Google Scholar] [CrossRef]
- Posgai, A.L.; Wasserfall, C.H.; Kwon, K.C.; Daniell, H.; Schatz, D.A.; Atkinson, M.A. Plant-based vaccines for oral delivery of type 1 diabetes-related autoantigens: Evaluating oral tolerance mechanisms and disease prevention in nod mice. Sci. Rep. 2017, 7, 42372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Huang, Y.; Yin, Z.; Menassa, R.; Brandle, J.E.; Jevnikar, A.M. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (gad) and il-4. Proc. Natl. Acad. Sci. USA 2004, 101, 5680–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Liu, D.; Wang, D.; Wang, Y.; Fu, Q.; Fallon, J.K.; Yang, X.; He, Z.; Liu, F. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat mdr in cancer. Mol. Pharm. 2014, 11, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Autonell, I.; Mansilla, M.J.; Rodriguez-Fernandez, S.; Cano-Sarabia, M.; Navarro-Barriuso, J.; Ampudia, R.M.; Rius, A.; Garcia-Jimeno, S.; Perna-Barrull, D.; Martinez-Caceres, E.; et al. Liposome-based immunotherapy against autoimmune diseases: Therapeutic effect on multiple sclerosis. Nanomed. (Lond.) 2017, 12, 1231–1242. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Ebrahimimonfared, M.; Ganji, A.; Zahedi, S.; Nourbakhsh, P.; Ghasami, K.; Mosayebi, G. Characterization of regulatory t-cells in multiple sclerosis patients treated with interferon beta-1a. CNS Neurol. Disord. Drug Targets 2018, 17, 113–118. [Google Scholar] [CrossRef]
- Fernandez, O.; Arbizu, T.; Izquierdo, G.; Martinez-Yelamos, A.; Gata, J.M.; Luque, G.; de Ramon, E. Clinical benefits of interferon beta-1a in relapsing-remitting ms: A phase iv study. Acta Neurol. Scand. 2003, 107, 7–11. [Google Scholar] [CrossRef]
- The IFNB multiple sclerosis study group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993, 43, 655–661. [Google Scholar] [CrossRef]
- Paty, D.W.; Li, D.K.; Ubc ms/mri study group; the ifnb multiple sclerosis study group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. Mri analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993, 43, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Belogurov, A., Jr.; Zakharov, K.; Lomakin, Y.; Surkov, K.; Avtushenko, S.; Kruglyakov, P.; Smirnov, I.; Makshakov, G.; Lockshin, C.; Gregoriadis, G.; et al. Cd206-targeted liposomal myelin basic protein peptides in patients with multiple sclerosis resistant to first-line disease-modifying therapies: A first-in-human, proof-of-concept dose-escalation study. Neurotherapeutics 2016, 13, 895–904. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.L.S.; Rudin, S.; Chang, R.; Mitchell, K.; Crandall, T.; Huang, S.; Choi, J.K.; Okitsu, S.L.; Graham, D.L.; Tomkinson, B.; et al. Atx-ms-1467 induces long-term tolerance to myelin basic protein in (dr2 x ob1)f1 mice by induction of il-10-secreting itregs. Neurol. Ther. 2018, 7, 103–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbark, A.A. Tcr peptide vaccination in multiple sclerosis: Boosting a deficient natural regulatory network that may involve tcr-specific cd4+cd25+ treg cells. Curr. Drug Targets. Inflamm. Allergy 2005, 4, 217–229. [Google Scholar] [CrossRef]
- Rorsman, I.; Petersen, C.; Nilsson, P.C. Cognitive functioning following one-year natalizumab treatment: A non-randomized clinical trial. Acta Neurol. Scand. 2018, 137, 117–124. [Google Scholar] [CrossRef]
- Perumal, J.; Fox, R.J.; Balabanov, R.; Balcer, L.J.; Galetta, S.; Makh, S.; Santra, S.; Hotermans, C.; Lee, L. Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: A prespecified 2-year interim analysis of strive. BMC Neurol. 2019, 19, 116. [Google Scholar] [CrossRef]
- Kapoor, R.; Ho, P.R.; Campbell, N.; Chang, I.; Deykin, A.; Forrestal, F.; Lucas, N.; Yu, B.; Arnold, D.L.; Freedman, M.S.; et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ascend): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet. Neurol. 2018, 17, 405–415. [Google Scholar] [CrossRef]
- Ranger, A.; Ray, S.; Szak, S.; Dearth, A.; Allaire, N.; Murray, R.; Gardner, R.; Cadavid, D.; Mi, S. Anti-lingo-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies. Neurol. (R) Neuroimmunol. Neuroinflammation 2018, 5, e417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Bar-Or, A.; Grove, R.A.; Austin, D.J.; Tolson, J.M.; VanMeter, S.A.; Lewis, E.W.; Derosier, F.J.; Lopez, M.C.; Kavanagh, S.T.; Miller, A.E.; et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The mirror study. Neurology 2018, 90, e1805–e1814. [Google Scholar] [CrossRef]
- Bhargava, P.; Wicken, C.; Smith, M.D.; Strowd, R.E.; Cortese, I.; Reich, D.S.; Calabresi, P.A.; Mowry, E.M. Trial of intrathecal rituximab in progressive multiple sclerosis patients with evidence of leptomeningeal contrast enhancement. Mult. Scler. Relat. Disord. 2019, 30, 136–140. [Google Scholar] [CrossRef]
- Salzer, J.; Svenningsson, R.; Alping, P.; Novakova, L.; Bjorck, A.; Fink, K.; Islam-Jakobsson, P.; Malmestrom, C.; Axelsson, M.; Vagberg, M.; et al. Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy. Neurology 2016, 87, 2074–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naismith, R.T.; Piccio, L.; Lyons, J.A.; Lauber, J.; Tutlam, N.T.; Parks, B.J.; Trinkaus, K.; Song, S.K.; Cross, A.H. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: A 52-week phase ii trial. Neurology 2010, 74, 1860–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, J.; Burman, J.; Gilthorpe, J.D.; Zetterberg, H.; Jiltsova, E.; Bergenheim, T.; Svenningsson, A. Intrathecal treatment trial of rituximab in progressive ms: An open-label phase 1b study. Neurology 2018, 91, e1893–e1901. [Google Scholar] [CrossRef] [PubMed]
- Hawker, K.; O’Connor, P.; Freedman, M.S.; Calabresi, P.A.; Antel, J.; Simon, J.; Hauser, S.; Waubant, E.; Vollmer, T.; Panitch, H.; et al. Rituximab in patients with primary progressive multiple sclerosis: Results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009, 66, 460–471. [Google Scholar] [CrossRef]
- Mayer, L.; Kappos, L.; Racke, M.K.; Rammohan, K.; Traboulsee, A.; Hauser, S.L.; Julian, L.; Kondgen, H.; Li, C.; Napieralski, J.; et al. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized opera i, opera ii, and oratorio studies. Mult. Scler. Relat. Disord. 2019, 30, 236–243. [Google Scholar] [CrossRef]
- Turner, B.; Cree, B.A.C.; Kappos, L.; Montalban, X.; Papeix, C.; Wolinsky, J.S.; Buffels, R.; Fiore, D.; Garren, H.; Han, J.; et al. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J. Neurol. 2019, 266, 1182–1193. [Google Scholar] [CrossRef]
- Fox, E.J.; Markowitz, C.; Applebee, A.; Montalban, X.; Wolinsky, J.S.; Belachew, S.; Fiore, D.; Pei, J.; Musch, B.; Giovannoni, G. Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase iii randomized oratorio trial. Mult. Scler. 2018, 24, 1862–1870. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Johnson, K.P.; Brooks, B.R.; Ford, C.C.; Goodman, A.; Guarnaccia, J.; Lisak, R.P.; Myers, L.W.; Panitch, H.S.; Pruitt, A.; Rose, J.W.; et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 multiple sclerosis study group. Mult. Scler. 2000, 6, 255–266. [Google Scholar] [CrossRef]
- Ouspid, E.; Razazian, N.; Moghadasi, A.N.; Moradian, N.; Afshari, D.; Bostani, A.; Sariaslani, P.; Ansarian, A. Clinical effectiveness and safety of fingolimod in relapsing remitting multiple sclerosis in western iran. Neurosciences (Riyadh) 2018, 23, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Ntranos, A.; Hall, O.; Robinson, D.P.; Grishkan, I.V.; Schott, J.T.; Tosi, D.M.; Klein, S.L.; Calabresi, P.A.; Gocke, A.R. Fty720 impairs cd8 t-cell function independently of the sphingosine-1-phosphate pathway. J. Neuroimmunol. 2014, 270, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K.; Khatri, B.; Edwards, K.R.; Meca-Lallana, J.E.; Cavalier, S.; Rufi, P.; Benamor, M.; Poole, E.M.; Robinson, M.; Gold, R. Teriflunomide real-world evidence: Global differences in the phase 4 teri-pro study. Mult. Scler. Relat. Disord. 2019, 31, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.E.; Xu, X.; Macdonell, R.; Vucic, S.; Truffinet, P.; Benamor, M.; Thangavelu, K.; Freedman, M.S. Efficacy and safety of teriflunomide in asian patients with relapsing forms of multiple sclerosis: A subgroup analysis of the phase 3 tower study. J. Clin. Neurosci. 2019, 59, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, M.S.; Wolinsky, J.S.; Comi, G.; Kappos, L.; Olsson, T.P.; Miller, A.E.; Thangavelu, K.; Benamor, M.; Truffinet, P.; O’Connor, P.W. The efficacy of teriflunomide in patients who received prior disease-modifying treatments: Subgroup analyses of the teriflunomide phase 3 temso and tower studies. Mult. Scler. 2018, 24, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Saida, T.; Yamamura, T.; Kondo, T.; Yun, J.; Yang, M.; Li, J.; Mahadavan, L.; Zhu, B.; Sheikh, S.I. A randomized placebo-controlled trial of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis from east asia and other countries. BMC Neurol. 2019, 19, 5. [Google Scholar] [CrossRef]
- Berger, T.; Brochet, B.; Brambilla, L.; Giacomini, P.S.; Montalban, X.; Vasco Salgado, A.; Su, R.; Bretagne, A. Effectiveness of delayed-release dimethyl fumarate on patient-reported outcomes and clinical measures in patients with relapsing-remitting multiple sclerosis in a real-world clinical setting: Protec. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319887191. [Google Scholar] [CrossRef] [Green Version]
- Rivera, V.M.; Jeffery, D.R.; Weinstock-Guttman, B.; Bock, D.; Dangond, F. Results from the 5-year, phase iv renew (registry to evaluate novantrone effects in worsening multiple sclerosis) study. BMC Neurol. 2013, 13, 80. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, D.L.; Ponda, P. Antigen-based immunotherapy for autoimmune disease: Current status. Immunotargets Ther. 2015, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chiba, K.; Yanagawa, Y.; Masubuchi, Y.; Kataoka, H.; Kawaguchi, T.; Ohtsuki, M.; Hoshino, Y. Fty720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. Fty720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 1998, 160, 5037–5044. [Google Scholar]
- Claussen, M.C.; Korn, T. Immune mechanisms of new therapeutic strategies in ms: Teriflunomide. Clin. Immunol. 2012, 142, 49–56. [Google Scholar] [CrossRef]
- Fox, E.J. Mechanism of action of mitoxantrone. Neurology 2004, 63, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Gold, R. Dimethyl fumarate for treatment of multiple sclerosis: Mechanism of action, effectiveness, and side effects. Curr. Neurol. Neurosci. Rep. 2013, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, W.J.; Kim, Y.H.; Lee, S.; Koo, J.H.; Lee, J.A.; Yoon, H.; Kim, D.H.; Park, H.J.; Kim, H.M.; et al. Dnp2 is a blood-brain barrier-permeable peptide enabling ctctla-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat. Commun. 2015, 6, 8244. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.H.; Kim, D.H.; Cha, D.; Kang, M.J.; Choi, J.M. Lrr domain of nlrx1 protein delivery by dnp2 inhibits t cell functions and alleviates autoimmune encephalomyelitis. Theranostics 2020, 10, 3138–3150. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Meka, R.R.; Venkatesha, S.H.; Lees, J.R.; Teesalu, T.; Moudgil, K.D. A novel cns-homing peptide for targeting neuroinflammatory lesions in experimental autoimmune encephalomyelitis. Mol. Cell. Probes 2020, 51, 101530. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N.; Barati, M.; Houshmand, F.; Hassani, S.; Clarner, T.; Shahlaei, M.; Golab, F. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of ampk/nrf2/mtor signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol. Rep. 2019. [Google Scholar] [CrossRef]
- Harada, M.; Kamimura, D.; Arima, Y.; Kohsaka, H.; Nakatsuji, Y.; Nishida, M.; Atsumi, T.; Meng, J.; Bando, H.; Singh, R.; et al. Temporal expression of growth factors triggered by epiregulin regulates inflammation development. J. Immunol. 2015, 194, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Severa, M.; Zhang, J.; Giacomini, E.; Rizzo, F.; Etna, M.P.; Cruciani, M.; Garaci, E.; Chopp, M.; Coccia, E.M. Thymosins in multiple sclerosis and its experimental models: Moving from basic to clinical application. Mult. Scler. Relat. Disord. 2019, 27, 52–60. [Google Scholar] [CrossRef]
| Treatment | Mode of Action | MS Type | Study Format (Number of Participants) | Clinical Outcomes | Adverse Effects | Administration Route | References |
|---|---|---|---|---|---|---|---|
| Interferons | |||||||
| Interferon-β1a * | reduces immature-transitional B cell subset/plasmablasts ratio, increases CD27- and CD27+IgM+ memory B cell subsets, enhances Tregs | RRMS | case-control study/multicenter, open-label, prospective clinical trial, phase 4 (96) | reduction in relapse rates, reduction in MRI measurement of disease, well tolerated | flu-like symptoms, asthenia, fever, malaise, fatigue, local pain at the injection site | intramuscular injection | [97,98] |
| Interferon-β1b * | reduces neuron inflammation | RRMS | multicenter, randomized, double-blind, placebo-controlled trial (372) | reduced ARR, and MRI lesions | lymphopenia, skin reactions to injection, flu-like symptoms, fever, chills, myalgia, sweating, malaise | subcutaneous injection | [99,100] |
| Peptides | |||||||
| Peptide loaded cells | |||||||
| Myelin peptides (MOG1-20, MOG35-55, MBP13-32, MBP83-99, MBP111-129, MBP146-170, PLP139-154) | myelin peptide coupled autologous peripheral blood mononuclear cells, slightly increase T regulatory cells | RRMS SPMS | open-label, single-center, dose-escalation study, phase 1 trial (9) | safe and well tolerated | metallic flavor during infusion and IARs (diarrhea, headache, diverticulitis of sigma, neck pain, vision disturbance, dysesthesia, cold, gastric pain) | infusion | [41] |
| Peptide vaccines | |||||||
| NBI-5788 | altered MBP83-99 peptide, induces Th2-like cells APL-reactive | PPMS SPMS RRMS | multicenter phase 1 trial (11) | induced NBI-5788 responsive T cells, no clinical exacerbations | - | subcutaneous infusion | [39] |
| Xemys | mannosylated liposomes encapsulating MBP peptides, increases TNF-α, cytokine’s levels normalization | RRMS SPMS | phase 1 trial (18)/phase 1, open-label, dose-escalating, proof-of-concept study (20) | increased TNF-α serum levels, safe and well tolerated | injection site reaction, rhinitis, general weakness | subcutaneous infusion | [40,101] |
| peptides MBP85-99, MOG35-55, and PLP139-155 | induce T regs producing IL-10, reduce IFN-γ and TGF-β | RRMS | double-blind, placebo-controlled cohort study (30) | reduced GdE lesions and ARR | local skin reaction (redness, itching), upper respiratory tract infection, lacrimation | transdermally, with skin patch | [36,37] |
| ATX-MS-1467 | peptide mixture of MBP derived epitopes, induces MBP tolerance and IL-10 secreting T regs | RMS | multicenter, phase 1b (43), phase 2a, multicenter, single-arm trial (37) | reduced GdE lesions | erythema, induration, pain, pruritus, hemorrhage, alopecia, diarrhea | intradermal/ subcutaneous injection | [38,102] |
| DNA vaccine | |||||||
| BHT-3009 | decreases T cells | RRMS | randomized, multicenter, double-blind, placebo-controlled dose escalation, phase 1/2 trial (30)/randomized, placebo-controlled, phase 2 trial (289) | reduced GdE lesions, reduced myelin-specific autoantibodies, safe and well tolerated | infections, musculoskeletal, urinary, gastrointestinal psychiatric, respiratory effects (IARs) | intramuscular injections | [47,48] |
| TCR vaccines | |||||||
| TCR V beta 5.2, 39-59 and V beta 6.1, 39-59 | induce T regs | PMS | dose escalation study (11) | induced T cell immunity to synthetic peptides, safe | skin hypersensitivity reaction to the injection, no side effects or broad immunosuppression | intradermal injection | [65,103] |
| vβ5.2-38-58 | induce Th2 cells and inhibits MBP-specific Th1 cells | PMS | double-blind (23) | induced T cell immunity to synthetic peptides, attenuated disease progression | no side effects or broad immunosuppression | intradermal injection | [66] |
| BV5S2, BV6S5 and BV13S1 | induce IL-10 secreting T cells | RRM PMS | single-arm, open-label study (23) | induced T cell immunity to synthetic peptides, stabilized disease, improved FoxP3 expression, safe | no side effects | intramuscular injection | [67] |
| Monoclonal antibodies | |||||||
| Natalizumab * | anti-a4-integrin Ab, prevents leukocytes crossing BBB | early RRMS | controlled, non-randomized trial (34)/multicenter, observational, open-label, single-arm, phase 4 study (222) | reduced relapse rates, MRI lesions and progression of disability, improvement in information processing speed, NEDA, SDMT and MSIS-29 physical, psychological and quality-of-life | suicide attempt, acute kidney injury, anaphylactic reactions, bronchial obstruction, clostridium difficile colitis, conversion disorder, hydronephrosis, hyperkaliemia, hypotension, ileus, melanoma recurrent, migraine | intravenous infusion | [104,105] |
| SPMS | randomized, double-blind, placebo-controlled, phase 3 trial (889), open-label extension (291) | reduced progression of disability, improved ARR and MRI measurements, well tolerated | urinary tract infection, nasopharyngitis, fall, MS relapse, headache, fatigue, upper respiratory tract infection, back pain, arthralgia, pain in hands and feet, muscular weakness (IARs) | intravenous infusion | [106] | ||
| Opicinumab | anti-LINGO-1 Ab, allows oligodendricy maturation | RRMS SPMS | double-blind, dose-ranging, proof-of-concept, phase 2b study (418)/phase 1, randomized, multiple ascending dose study | primary endpoint was not met, inverted U-shaped dose-response | unaffected immune function | intravenous infusion | [81,107] |
| Alemtuzumab* | anti-CD52 IgG Ab, depletes circulating T and B lymphocytes | RRMS | rater-masked, randomized, controlled phase 3 trial (667) | reduced ARR, stabilized disability levels, improved clinical and MRI outcomes, reduced brain volume loss | infections, thyroid-associated adverse events, thrombocytopenia IARs (headache, pyrexia, rash, bradycardia, insomnia, erythema, nausea, Urticaria, pruritus, abdominal pain, fatigue, dyspnea, flushing) | intravenous infusion | [108] |
| Ofatumumab | anti-CD20, cytotoxic to B lymphocytes | RRMS | randomized, double-blind, placebo-controlled, phase 2 study (36)/randomized, double-blind, phase 2b study (232) | decreased new MRI lesions, safe | rash, erythema, upper respiratory tract infection, viral infection, throat irritation, headache, fatigue, back pain, flushing, injection related reactions | subcutaneous injection | [79,109] |
| Rituximab | selective depletion of CD20+ B lymphocytes | PMS | single-center, open-label trial (8)/retrospective, uncontrolled, observational, multicenter study (822) | reduced peripheral B cells, CSF B cells and CXCL-13 levels, increased BAFF levels/ lower EDSS score, delayed CDP | IARs (lower extremity paresthesia), lower extremity spasticity or weakness, fatigue, fever, rigors/ infections (respiratory, intestinal), disorders (cardiac, respiratory, neuronal, immune) and IARs (malaise, headache, chills, nausea) | intrathecal infusion | [110,111] |
| RRMS | blind, single-center, phase 2 trial (30) | reduced relapses and GdE lesions | IARs (fever, chills, flushing, itching of body or throat, and/or diarrhea, shortness of breath), urinary tract infections, thigh pain, upper respiratory tract infection, bronchitis, hand tendonitis, dizziness | intravenous infusion | [112] | ||
| PPMS SPMS | multicenter, prospective, open-label phase 1b trial (23)/randomized, double-blind, placebo-controlled, multicenter, phase 2/3 trial (439) | well tolerated and feasible, reduced GdE lesions, delayed CDP | IARs (vertigo, nausea), infections, paresthesia, fall, nervous system disorders, fever, fatigue, meningitis/IARs (nausea, fatigue, chills, pyrexia, headache, dizziness, throat irritation, pharyngolaryngeal pain, pruritus, rash, flushing, hypotension), pneumonia, bronchitis | intravenous or intrathecal infusion | [113,114] | ||
| Ocrelizumab* | anti-CD20 Ab, depletes circulating CD20+ B cells | RMS PPMS | randomized, double-blind, active-controlled, phase 3 trials (1651), randomized, parallel-group, double-blind, placebo- controlled, phase 3 study (725) | reduced new and GdE lesions, improved ARR, disability progression, and MRI outputs | IARs (pruritus, rash, throat irritation, flushing, urticaria, oropharyngeal pain, headache, tachycardia, pyrexia, nausea, hypo-, hyper-tension, myalgia, dizziness, fatigue) | intravenous infusion | [115,116] |
| PPMS | randomized, double-blind, placebo-controlled, phase 3 trial (732) | reduced risk of Upper Extremity disability progression, enhanced NEPAD, reduced brain volume loss | IARs (upper respiratory tract infections, oral herpes infections, pruritus, rash, throat irritation, flushing) | intravenous infusion | [117,118] | ||
| HLA antagonistic co-polymers | |||||||
| Glatiramer acetate * | increases Tregs to suppress inflammatory response | RRMS | randomized, placebo-controlled, double-blind study (251), open-label (208) | reduced relapse rate, reduced GdE and new lesions | IARs (flushing, anxiety, dyspnea) | subcutaneous injection | [119] |
| Sphingosine-1-phosphate receptor modulators | |||||||
| Fingolimod * | structural analogue of sphingosine, anti-inflammatory, impairs cytotoxic CD8 T cells function | RRMS | prospective observational study (60) | higher retention rate, increased satisfaction at MSQ, reduced dGM volume loss, ARR and EDSS | influenza-like illness, pain in extremity, headache, anxiety, depression, nasopharyngitis, hypoesthesia, arthralgia, dizziness, fatigue, rash, urinary tract infection, abdominal pain, hypertension, lymphopenia | oral | [120,121] |
| Other inhibitors | |||||||
| Teriflunomide * | DHODH inhibitor, reduces proliferation of T- and B-cells | RMS | prospective, single-arm, open-label, phase 4 real-world study (1000)/randomized, double-blind, placebo-controlled, phase 3 trial (168)/multicenter, multinational, randomized, double-blind, parallel-group, placebo-controlled, phase 3 study (2251) | well tolerated, improved MRI outcomes, reduced ARR and CDW, improved TSQM scores, stabilized disability measures, improved cognition and quality of life measures | neutropenia, hair thinning, diarrhea, nausea, headache, urinary tract infection, increased alanine aminotransferase, nasopharyngitis, fatigue, paresthesia | oral | [122,123,124] |
| T cell vaccination | |||||||
| MBP-reactive T cells | deplete circulating MBP-reactive T cells. | RRMSSPMS | pilot, controlled (8)/preliminary open label study (54) | safe and well tolerated, improved MRI outcome, reduced relapse rates | no adverse effects, skin infection | subcutaneous injection | [52,53] |
| MBB-, MOG-reactive T cells | deplete circulating MBP-, MOG-reactive T cells. | RRMS | 20 | improved MRI outcome | no adverse effects, skin infection | S subcutaneous injection | [55] |
| MBP-, MOG-, PLP-reactive T cells/ Tovaxin | deplete circulating MBP-, MOG-, PLP-reactive T cells. | RRMSSPMS | open-label dose escalation study (16)/randomized, double-blind trial, phase 2 study (26) | well tolerated, reduced EDSS, ARR and 10 min walking time, stabilized MRI lesions, improved EDSS and MSIS-29 | relapse of MS, pain in extremity, IARs (injection site pain, erythema, inflammation, pruritus), unrelated to TCV administration (anemia, intestinal obstruction, pneumonia, carpal tunnel syndrome, headache, respiratory distress, infections) | Subcutaneous injection | [54,56] |
| Dendritic cell vaccination | |||||||
| peptide loaded cells | increase T regulatory cells and IL-10 levels | RRMSSPMSPPMS | open-label, single-center, multiple ascending-dose, phase 1b trial (12) | well tolerated, stabilized disease progress | headache, leg pain, cold, palpitations, influenza (and unrelated to TCV administration) | intravenous | [61] |
| Esters | |||||||
| Dimethyl Fumarate * | fumaric acid ester, modulates CD4(+) cells, M2 monocytes and B-cells, induction of antioxidant response | RRMS | randomized, double-blind, placebo controlled, phase 3 trial (213)/open-label, observational, phase 4 study (1105) | decreased EDSS, GdE and new lesions, reduced ARR, improved treatment satisfaction and quality of life measures | flushing, nausea, abdominal pain, diarrhea, gastrointestinal events, nasopharyngitis, infections, cardiovascular, skin and hepatic events, pruritus, rash, headache, fall, lymphopenia, breast cancer, MS relapse | oral delayed release | [125,126] |
| Other Immunomodulators | |||||||
| Mitoxantrone * | a synthetic anthracenedione, inhibits T-cell, B-cell and macrophage proliferation | SPMS RRMS PRMS | multicenter, prospective, open-label, observational, phase 4 study (509) | reduced GdE lesions and relapse rate, improved EDSS | congestive heart failure, leukemia, amenorrhea, decreased ejection fraction, urinary tract infection | intravenous infusion | [127] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metaxakis, A.; Petratou, D.; Tavernarakis, N. Molecular Interventions towards Multiple Sclerosis Treatment. Brain Sci. 2020, 10, 299. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10050299
Metaxakis A, Petratou D, Tavernarakis N. Molecular Interventions towards Multiple Sclerosis Treatment. Brain Sciences. 2020; 10(5):299. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10050299
Chicago/Turabian StyleMetaxakis, Athanasios, Dionysia Petratou, and Nektarios Tavernarakis. 2020. "Molecular Interventions towards Multiple Sclerosis Treatment" Brain Sciences 10, no. 5: 299. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10050299