A Multiple N-Glucosylated Peptide Epitope Efficiently Detecting Antibodies in Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the N-Glc Peptides
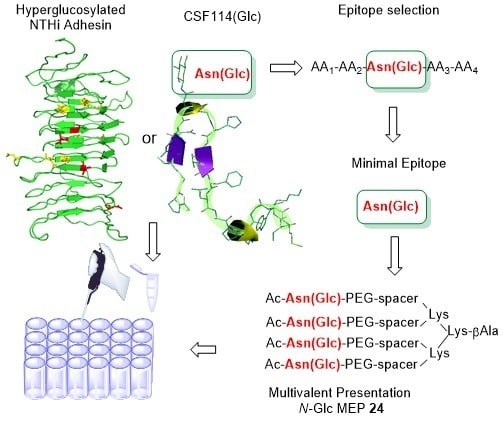
2.2. Synthesis of N-Glc Multiple Epitope Peptides (N-Glc MEPs)
2.3. Immunological Assays
2.3.1. Inhibition ELISA
2.3.2. Solid-Phase ELISA (SP-ELISA)
2.4. Statistical Analysis
3. Results
3.1. Antibody Detection in Solid-Phase ELISA (SP-ELISA) Is Affected by the Length of the Peptide Antigen
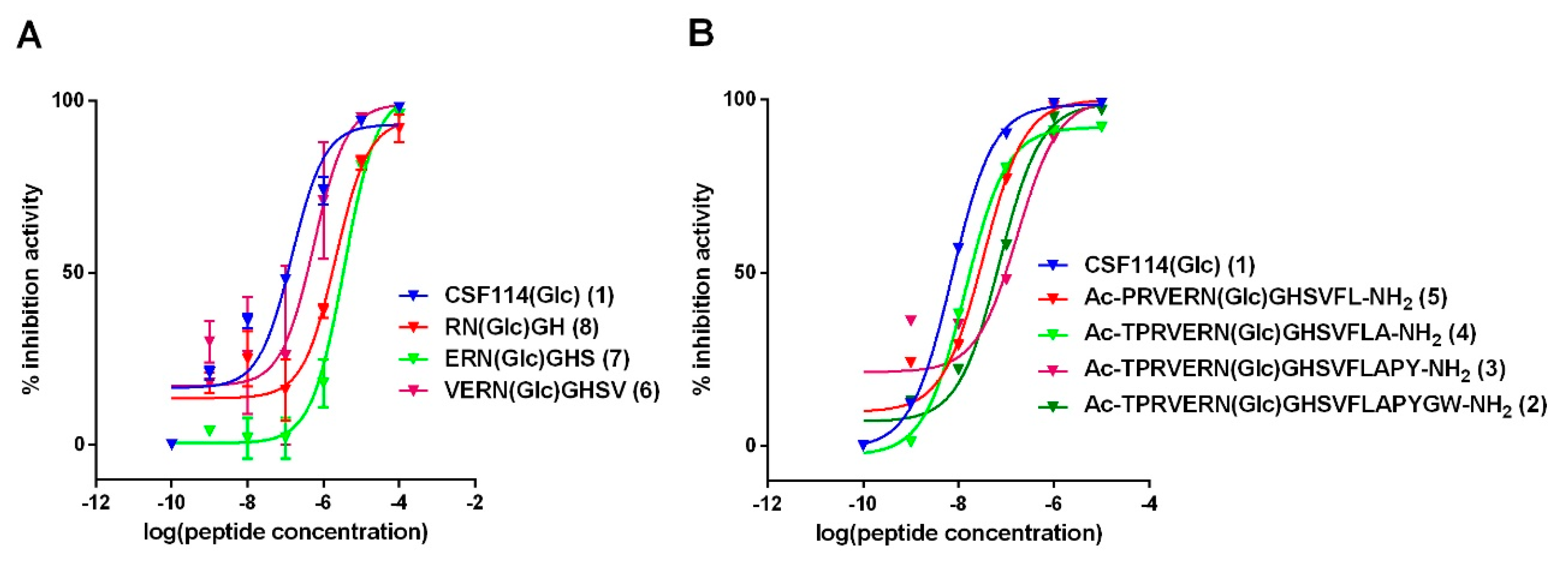
3.2. Antibody Affinity of Shortened CSF114(Glc) Glucopeptide Sequences in Competitive ELISA: Shortening CSF114(Glc) Does not Affect Antibody Epitope Recognition
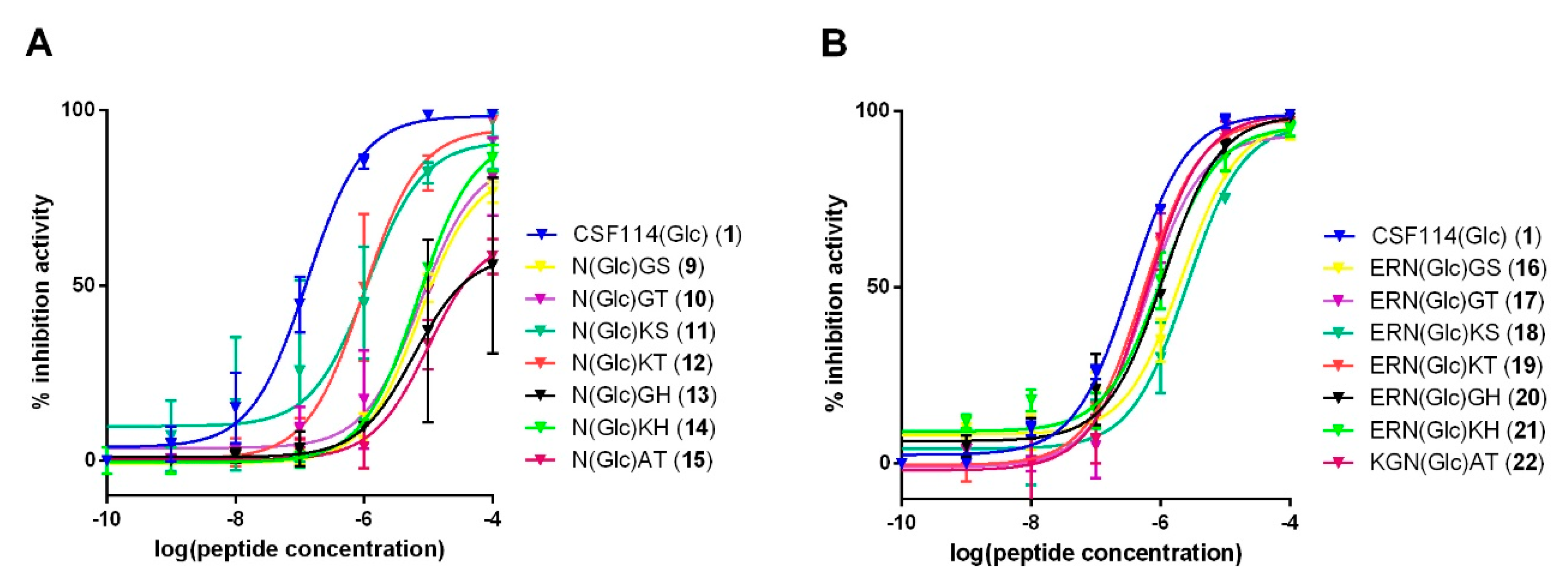
3.3. Study of the Possible Role of a Consensus Sequence Surrounding N(Glc) in Antibody Recognition
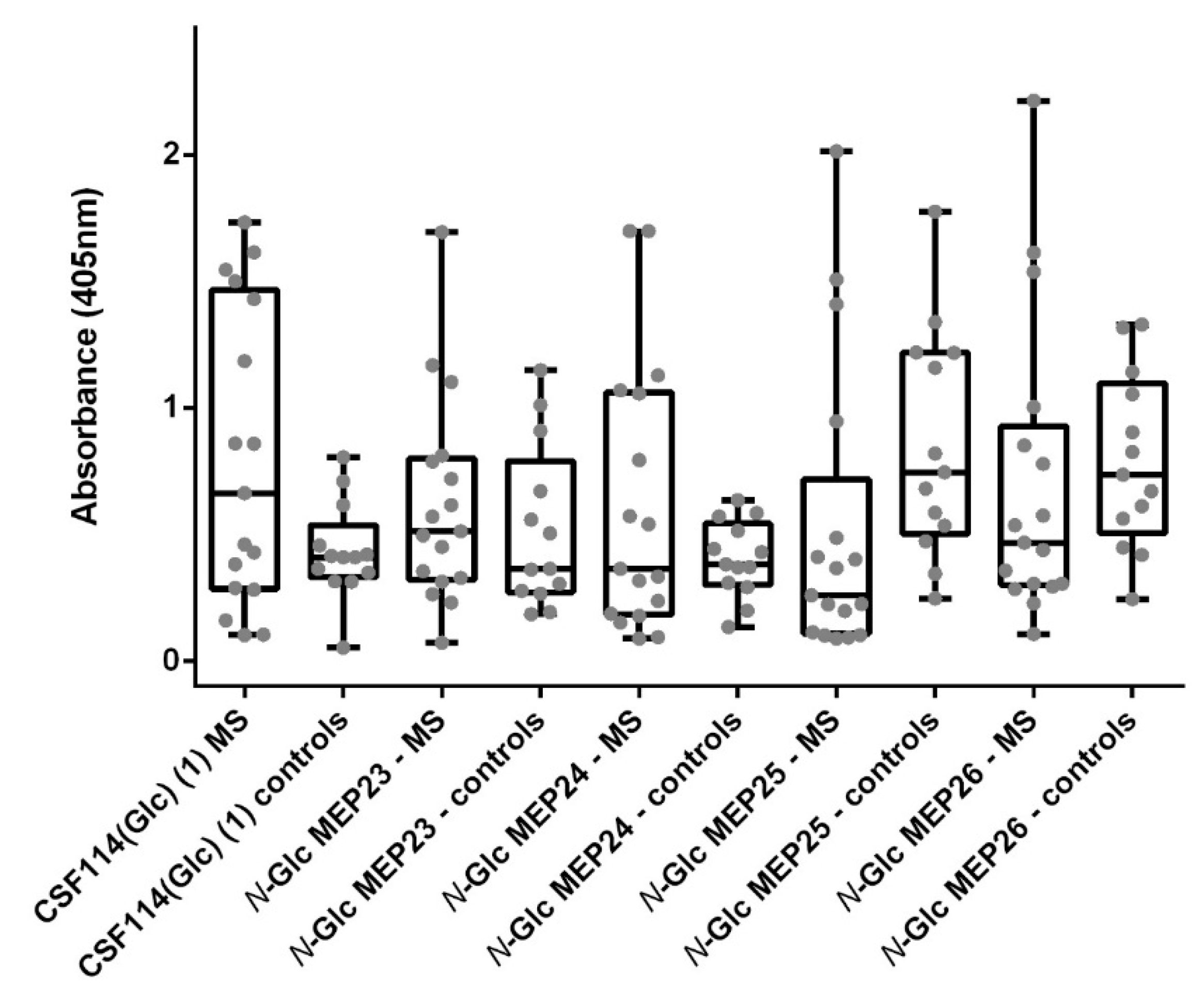
3.4. N-Glc Multiple Epitope Peptides (N-Glc MEPs) to Mimic Multivalency in SP-ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Seze, J.; Vermersch, P. Sequential magnetic resonance imaging follow-up of multiple sclerosis before the clinical phase. Mult. Scler. 2005, 11, 395–397. [Google Scholar] [CrossRef]
- Lanzillo, R.; Prinster, A.; Scarano, V.; Liuzzi, R.; Coppola, G.; Florio, C.; Salvatore, E.; Schiavone, V.; Brunetti, A.; Muto, M.; et al. Neuropsychological assessment, quantitative MRI and ApoE gene polymorphisms in a series of MS patients treated with IFN beta-1b. J. Neurol. Sci. 2006, 245, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.M.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 62, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque, F.A.; Jaffe, S.L. Cerebrospinal fluid analysis in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 341–356. [Google Scholar] [PubMed]
- Reindl, M.; Jarius, S.; Rostasy, K.; Berger, T. Myelin oligodendrocyte glycoprotein antibodies: How clinically useful are they? Curr. Opin. Neurol. 2017, 30, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.J.; Komorowski, L.; Woodhall, M.; Lederer, S.; Majed, M.; Fryer, J.; Mills, J.; Flanagan, E.P.; Irani, S.R.; Kunchok, A.C. A multicenter comparison of MOG-IgG cell-based assays. Neurology 2019, 92, e1250–e1255. [Google Scholar] [CrossRef]
- Papp, V.; Langkilde, A.R.; Blinkenberg, M.; Schreiber, K.; Jensen, P.E.H.; Sellebjerg, F. Clinical utility of anti-MOG antibody testing in a Danish cohort. Mult. Scler. Relat. Disord. 2018, 26, 61–67. [Google Scholar] [CrossRef]
- de Seze, J. Myelin oligodendrocyte glycoprotein antibodies in neuromyelitis optica spectrum disorder. Curr. Opin. Neurol. 2019, 32, 111–114. [Google Scholar]
- Meloen, R.H.; Puijk, W.C.; Langeveld, J.P.; Langedijk, J.P.; Timmerman, P. Design of synthetic peptides for diagnostics. Curr. Protein Pept. Sci. 2003, 4, 253–260. [Google Scholar] [CrossRef]
- Papini, A.M. The use of post-translationally modified peptides for detection of biomarkers of immune-mediated diseases. J. Pept. Sci. 2009, 15, 621–628. [Google Scholar] [CrossRef]
- Ballew, J.T.; Murray, J.A.; Collin, P.; Mäki, M.; Kagnoff, M.F.; Kaukinen, K.; Daugherty, P.S. Antibody biomarker discovery through in vitro directed evolution of consensus recognition epitopes. Proc. Natl. Acad. Sci. USA 2013, 110, 19330–19335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómara, M.J.; Fernández, L.; Pérez, T.; Ercilla, G.; Haro, I. Assessment of synthetic chimeric multiple antigenic peptides for diagnosis of GB virus C infection. Anal. Biochem. 2010, 396, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; Monsó, M.; Muñoz, M.; Rosell, R.; Fraile, L.; Frías, M.T.; Domingo, M.; Andreu, D.; Sobrino, F.; Ganges, L. Partial protection against classical swine fever virus elicited by dendrimeric vaccine-candidate peptides in domestic pigs. Vaccine 2011, 29, 4422–4429. [Google Scholar] [CrossRef]
- Buus, S.; Rockberg, J.; Forsström, B.; Nilsson, P.; Uhlen, M.; Schafer-Nielsen, C. High-resolution mapping of linear antibody epitopes using ultrahigh-density peptide microarrays. Mol. Cell. Proteom. 2012, 11, 1790–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebbes, T.R.; Turner, C.H.; Thorne, A.W.; Crane-Robinson, C. A “minimal epitope” anti-protein antibody that recognises a single modified amino acid. Mol. Immunol. 1989, 26, 865–873. [Google Scholar] [CrossRef]
- Waritani, T.; Chang, J.; McKinney, B.; Terato, K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 2017, 4, 153–165. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. Engl. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhou, L.; Lakshminarayanan, R.; Beuerman, R.W. Multivalent Antimicrobial Peptides as Therapeutics: Design Principles and Structural Diversities. Int. J. Pept. Res. Ther. 2010, 16, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Joshi, V.G.; Dighe, V.D.; Thakuria, D.; Malik, Y.S.; Kumar, S. Multiple antigenic peptide (MAP): A synthetic peptide dendrimer for diagnostic, antiviral and vaccine strategies for emerging and re-emerging viral diseases. Indian J. Virol. 2013, 24, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, J.P.; Zavala, F. Multiple antigen peptide. A novel approach to increase detection sensitivity of synthetic peptides in solid-phase immunoassays. J. Immunol. Methods 1989, 124, 53–61. [Google Scholar] [CrossRef]
- Doyle, H.A.; Mamula, M.J. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001, 22, 443–449. [Google Scholar] [CrossRef]
- Lolli, F.; Mazzanti, B.; Pazzagli, M.; Peroni, E.; Alcaro, M.C.; Sabatino, G.; Lanzillo, R.; Brescia Morra, V.; Santoro, L.; Gasperini, C.; et al. The glycopeptide CSF114(Glc) detects serum antibodies in multiple sclerosis. J. Neuroimmunol. 2005, 167, 131–137. [Google Scholar] [CrossRef]
- Lolli, F.; Mulinacci, B.; Carotenuto, A.; Bonetti, B.; Sabatino, G.; Mazzanti, B.; D’Ursi, A.M.; Novellino, E.; Pazzagli, M.; Lovato, L.; et al. An N-glucosylated peptide detecting disease-specific autoantibodies, biomarkers of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2005, 102, 10273–10278. [Google Scholar] [CrossRef] [Green Version]
- Walvoort, M.T.; Testa, C.; Eilam, R.; Aharoni, R.; Nuti, F.; Rossi, G.; Lanzillo, R.; Papini, A.M.; Imperiali, B.; Rovero, P.; et al. Antibodies from multiple sclerosis patients preferentially recognize hyperglucosylated adhesin of non-typeable Haemophilus influenzae. Sci. Rep. 2017, 7, 44969. [Google Scholar] [CrossRef] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of multiple sclerosis: 2017 revisions of the Mc Donald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Rath, S.; Stanley, C.M.; Steward, M.W. An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J. Immunol. Methods 1988, 106, 245–249. [Google Scholar] [CrossRef]
- Real Fernández, F.; Di Pisa, M.; Rossi, G.; Auberger, N.; Lequin, O.; Larregola, M.; Benchohra, A.; Mansuy, C.; Chessaing, G.; Lolli, F.; et al. Antibody recognition in multiple sclerosis and Rett syndrome using a collection of linear and cyclic N-glucosylated antigenic probes. Biopolymers 2015, 104, 560–576. [Google Scholar] [CrossRef]
- Schwarz, F.; Fan, Y.Y.; Schubert, M.; Aebi, M. Cytoplasmic N-glycosyltransferase of Actinobacillus pleuropneumoniae is an inverting enzyme and recognizes the NX(S/T) consensus sequence. J. Biol. Chem. 2011, 286, 35267–35274. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.J.; Grass, S.; Paek, S.; St Geme, J.W.; Yeo, H.J. The Actinobacillus pleuropneumoniae HMW1C-like glycosyltransferase mediates N-linked glycosylation of the Haemophilus influenzae HMW1 adhesin. PLoS ONE 2010, 5, e15888. [Google Scholar] [CrossRef] [Green Version]
- Carotenuto, A.; D’Ursi, A.M.; Mulinacci, B.; Paolini, I.; Lolli, F.; Papini, A.M.; Novellino, E.; Rovero, P. Conformation-activity relationship of designed glycopeptides as synthetic probes for the detection of autoantibodies, biomarkers of multiple sclerosis. J. Med. Chem. 2006, 49, 5072–5079. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H.V. Antigenicity and Immunogenicity of Synthetic Peptides. Biologicals 2001, 29, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, S.; Matà, S.; Vergelli, M.; Fioresi, R.; Nardi, E.; Mazzanti, B.; Chelli, M.; Lolli, F.; Giannenschi, M.; Pinto, F.; et al. Synthetic glycopeptide of human myelin oligodendrocyte glycoprotein to detect antibody responses in multiple sclerosis and other neurological diseases. Bioorg. Med. Chem. Lett. 1999, 9, 167–172. [Google Scholar] [CrossRef]
- Araújo, P.R.; Ferreira, A.W. High diagnostic efficiency of IgM-ELISA with the use of multiple antigen peptides (MAP1) from T. gondii ESA (SAG-1, GRA-1 and GRA-7), in acute toxoplasmosis. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolini, I.; Nuti, F.; de la Cruz Pozo-Carrero, M.; Barbetti, F.; Kolesinska, B.; Kaminski, Z.J.; Chelli, M.; Papini, A.M. A convenient microwave-assisted synthesis of N-glycosyl amino acids. Tetrahedron Lett. 2007, 48, 2901–2904. [Google Scholar] [CrossRef]
- Habbema, J.D.; Eijkemans, R.; Krijnen, J.; Knottnerus, J.E. Analysis of data on the accuracy of diagnostic tests. In The Evidence Base of Clinical Diagnosis; Knottnerus, J.E., Ed.; BMJ Books: London, UK, 2002; pp. 117–143. [Google Scholar]
- Calabrese, M.; Gasperini, C.; Tortorella, C.; Schiavi, G.; Frisullo, G.; Ragonese, P.; Fantozzi, R.; Prosperini, L.; Annovazzi, P.; Cordioli, C.; et al. “Better explanations” in multiple sclerosis diagnostic workup: A 3-year longitudinal study. Neurology 2019, 92, e2527–e2537. [Google Scholar] [CrossRef] [Green Version]
- Migliorini, P.; Pratesi, F.; Tommasi, C.; Anzilotti, C. The immune response to citrullinated antigens in autoimmune diseases. Autoimmun. Rev. 2005, 4, 561–564. [Google Scholar] [CrossRef]
- Gestwicki, J.E.; Cairo, C.W.; Strong, L.E.; Oetjen, K.A.; Kiessling, L.L. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 2002, 124, 14922–14933. [Google Scholar] [CrossRef]
- Sadler, K.; Tam, J.P. Peptide dendrimers: Applications and synthesis. J. Biotechnol. 2002, 90, 195–229. [Google Scholar] [CrossRef]
- Weimer, B.C.; Walsh, M.K.; Wang, X. Influence of a poly-ethylene glycol spacer on antigen capture by immobilized antibodies. J. Biochem. Biophys. Methods 2000, 45, 211–219. [Google Scholar] [CrossRef]



| Peptide Name Number | N-Glucosylated Peptide Sequence |
|---|---|
| CSF114(Glc) (1) | TPRVERN(Glc)GHSVFLAPYGWMVK |
| [Asn7(Glc)] CSF114(1–18) (2) | Ac-TPRVERN(Glc)GHSVFLAPYGW-NH2 |
| [Asn7(Glc)] CSF114(1–16) (3) | Ac-TPRVERN(Glc)GHSVFLAPY-NH2 |
| [Asn7(Glc)] CSF114(1–14) (4) | Ac-TPRVERN(Glc)GHSVFLA-NH2 |
| [Asn7(Glc)] CSF114(2–13) (5) | Ac-PRVERN(Glc)GHSVFL-NH2 |
| [Asn7(Glc)] CSF114(4–11) (6) | Ac-VERN(Glc)GHSV-NH2 |
| [Asn7(Glc)] CSF114(5–10) (7) | Ac-ERN(Glc)GHS-NH2 |
| [Asn7(Glc)] CSF114(6–9) (8) | Ac-RN(Glc)GH-NH2 |
| Glucosylated Core Peptide | Glucosylated Tripeptides | Glucosylated Pentapeptides |
|---|---|---|
| Selected NXT/S sequences | Ac-N(Glc)GS-NH2 (9); Ac-N(Glc)GT-NH2 (10); Ac-N(Glc)KS-NH2 (11); Ac-N(Glc)KT-NH2 (12). | Ac-ERN(Glc)GS-NH2 (16); Ac-ERN(Glc)GT-NH2 (17); Ac-ERN(Glc)KS-NH2 (18); Ac-ERN(Glc)KT-NH2 (19) |
| CSF114(Glc) | Ac-N(Glc)GH-NH2 (13); Ac-N(Glc)KH-NH2 (14) | Ac-ERN(Glc)GH-NH2 (20); Ac-ERN(Glc)KH-NH2 (21) |
| [Asn31(Glc)]hMOG(30–50) | Ac-N(Glc)AT-NH2 (15) | Ac-KGN(Glc)AT-NH2 (22) |
| Compound | Area Under Curve (AUC) | Criterion Value and Coordinates | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC | Standard Error (SE) | 95% Confidence Interval | p-Value | Cut-Off | Sensitivity (%) | Specificity (%) | Likelihood Ratio | |
| N-Glc MEP (23) | 0.5747 | 0.1073 | 0.3643 to 0.7850 | 0.4899 | >1.057 | 17.65 (3.799 to 43.43) | 92.31 (63.97 to 99.81) | 2.294 |
| N-Glc MEP (24) | 0.5249 | 0.1100 | 0.3092 to 0.7406 | 0.8180 | >0.6104 | 35.29 (14.21 to 61.67) | 92.31 (63.97 to 99.81) | 4.588 |
| N-Glc MEP (25) | 0.7511 | 0.09376 | 0.5673 to 0.9350 | 0.02023 | >1.053 | 38.46 (13.86 to 68.42) | 82.35 (56.57 to 96.20) | 2.179 |
| N-Glc MEP (26) | 0.6380 | 0.1041 | 0.4339 to 0.8421 | 0.2018 | >1.029 | 30.77 (9.092 to 61.43) | 82.35 (56.57 to 96.20) | 1.744 |
| CSF114(Glc) (1) | 0.6516 | 0.1049 | 0.4458 to 0.8573 | 0.1610 | >0.7575 | 47.06 (22.98 to 72.19) | 92.31 (63.97 to 99.81) | 6.118 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuti, F.; Fernandez, F.R.; Sabatino, G.; Peroni, E.; Mulinacci, B.; Paolini, I.; Pisa, M.D.; Tiberi, C.; Lolli, F.; Petruzzo, M.; et al. A Multiple N-Glucosylated Peptide Epitope Efficiently Detecting Antibodies in Multiple Sclerosis. Brain Sci. 2020, 10, 453. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10070453
Nuti F, Fernandez FR, Sabatino G, Peroni E, Mulinacci B, Paolini I, Pisa MD, Tiberi C, Lolli F, Petruzzo M, et al. A Multiple N-Glucosylated Peptide Epitope Efficiently Detecting Antibodies in Multiple Sclerosis. Brain Sciences. 2020; 10(7):453. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10070453
Chicago/Turabian StyleNuti, Francesca, Feliciana Real Fernandez, Giuseppina Sabatino, Elisa Peroni, Barbara Mulinacci, Ilaria Paolini, Margherita Di Pisa, Caterina Tiberi, Francesco Lolli, Martina Petruzzo, and et al. 2020. "A Multiple N-Glucosylated Peptide Epitope Efficiently Detecting Antibodies in Multiple Sclerosis" Brain Sciences 10, no. 7: 453. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10070453