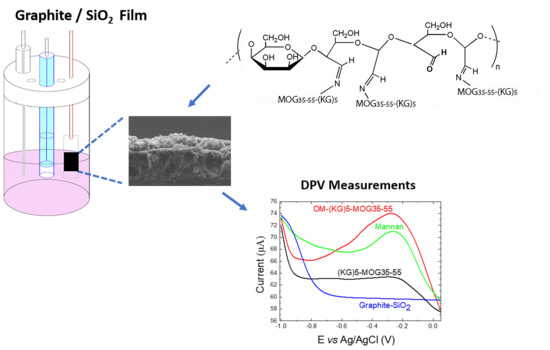
The Use of Electrochemical Voltammetric Techniques and High-Pressure Liquid Chromatography to Evaluate Conjugation Efficiency of Multiple Sclerosis Peptide-Carrier Conjugates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphite/SiO2 Film Electrodes Preparation
2.3. Characterization of Graphite/SiO2 Film Electrodes
2.4. Preparation of (KG)5-MOG35-55 Peptide
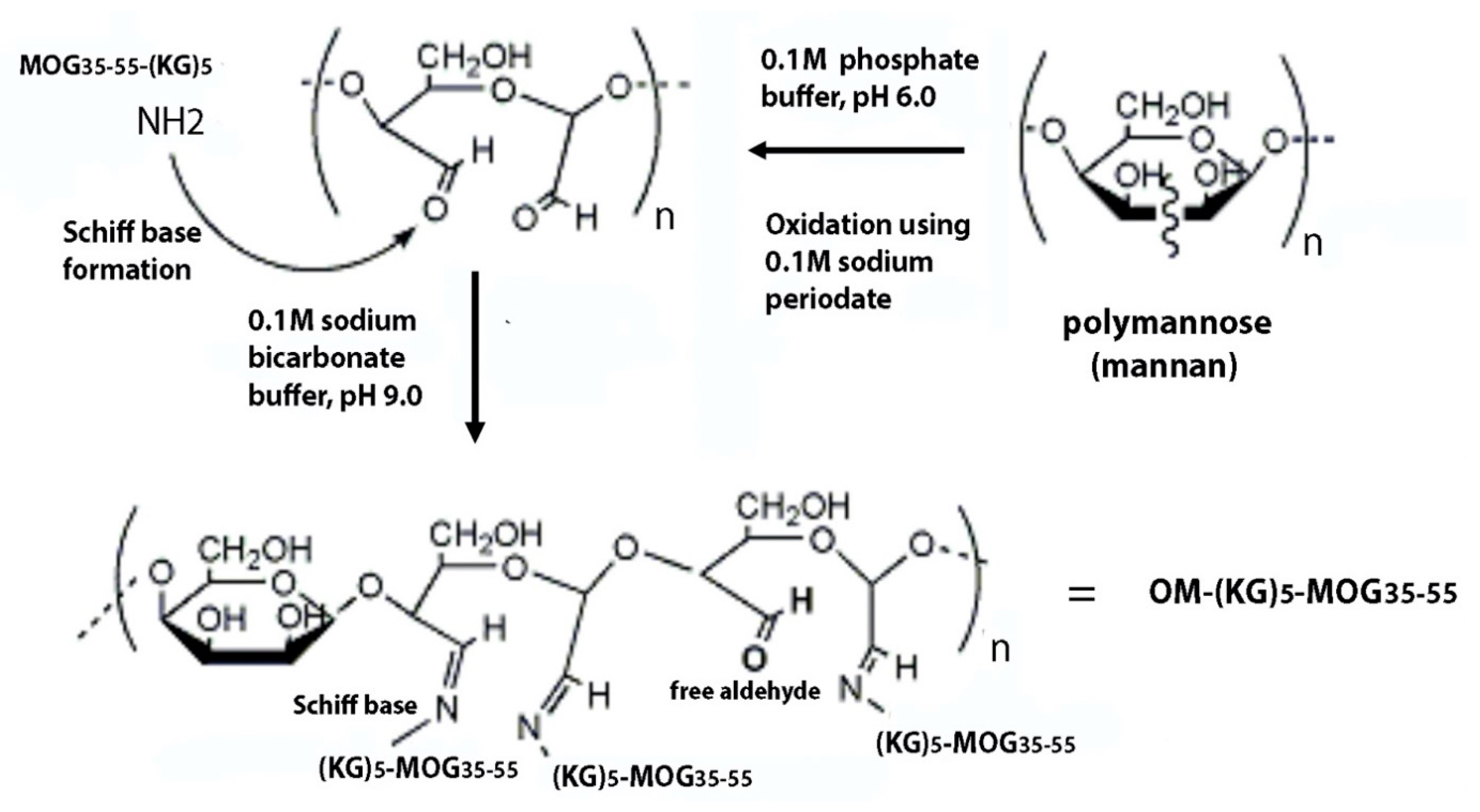
2.5. Preparation of Oxidized Mannan
2.6. Conjugation of Oxidized Mannan to Peptide
2.7. Monitoring of Conjugation by HPLC
2.8. Electrochemical/Electrocatalytic Measurements
3. Results and Discussion
3.1. FE-SEM Characterization
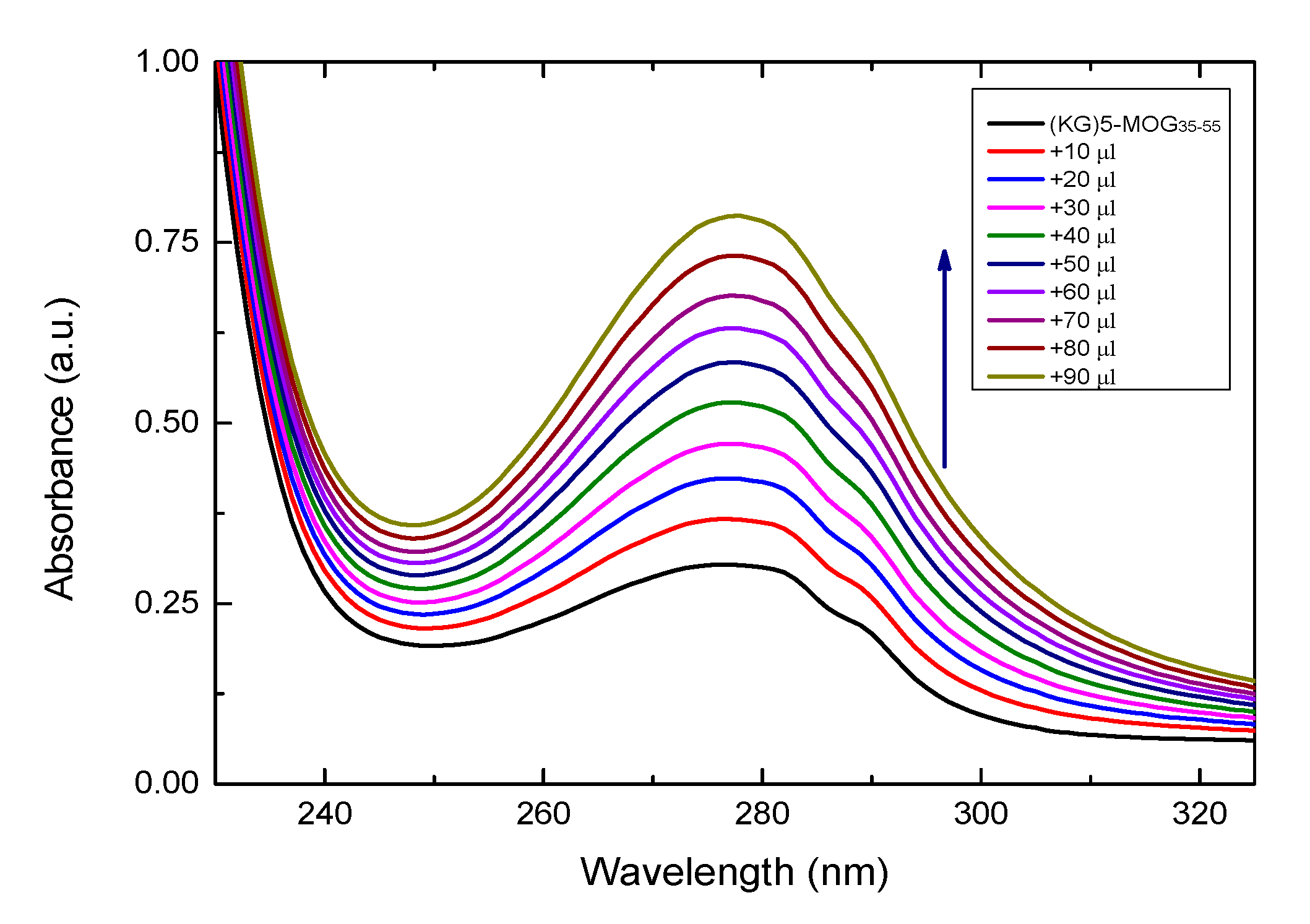
3.2. UV Characterazation of (KG)5-MOG35-55 Peptide with Increasing Amounts of OM
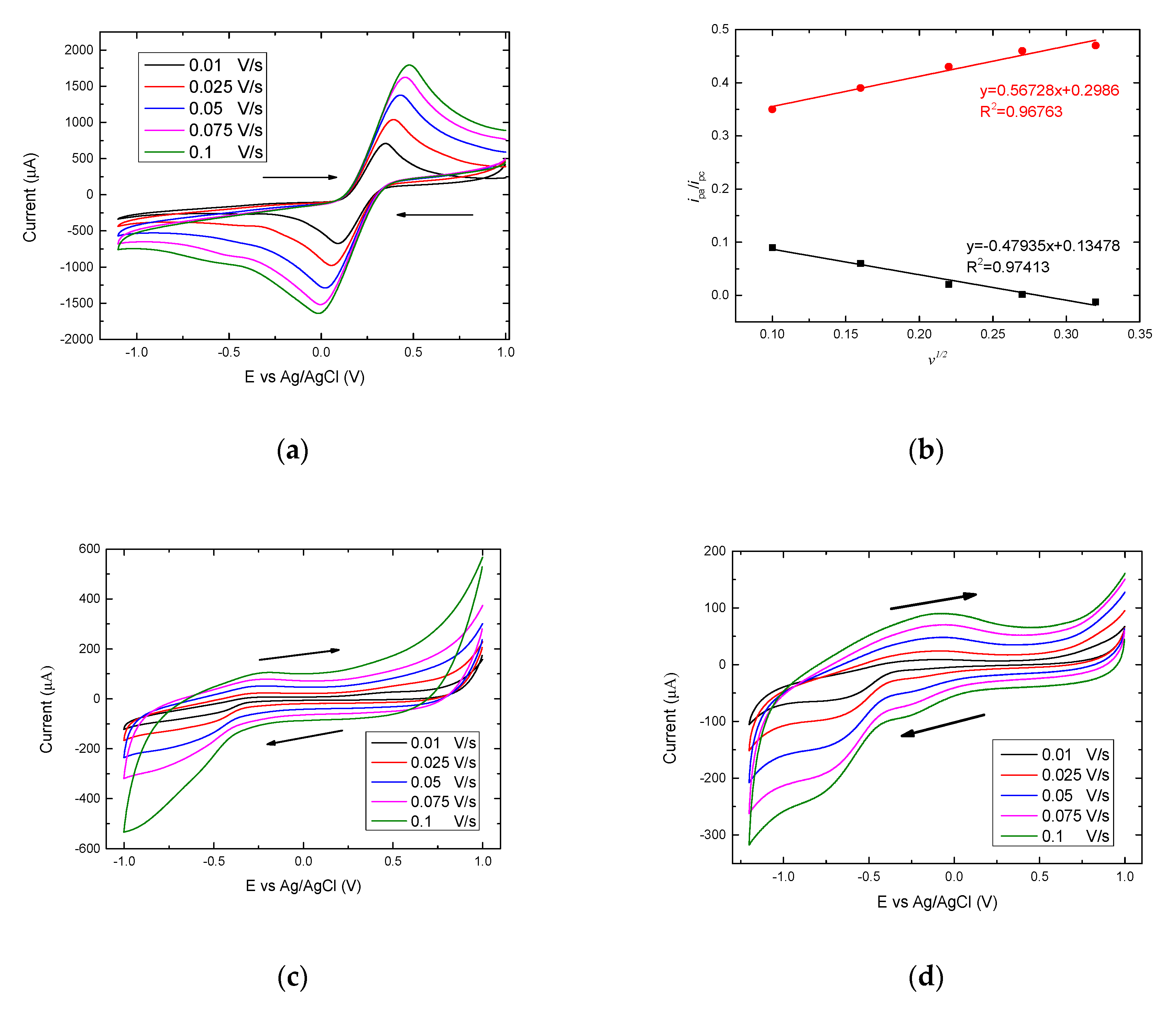
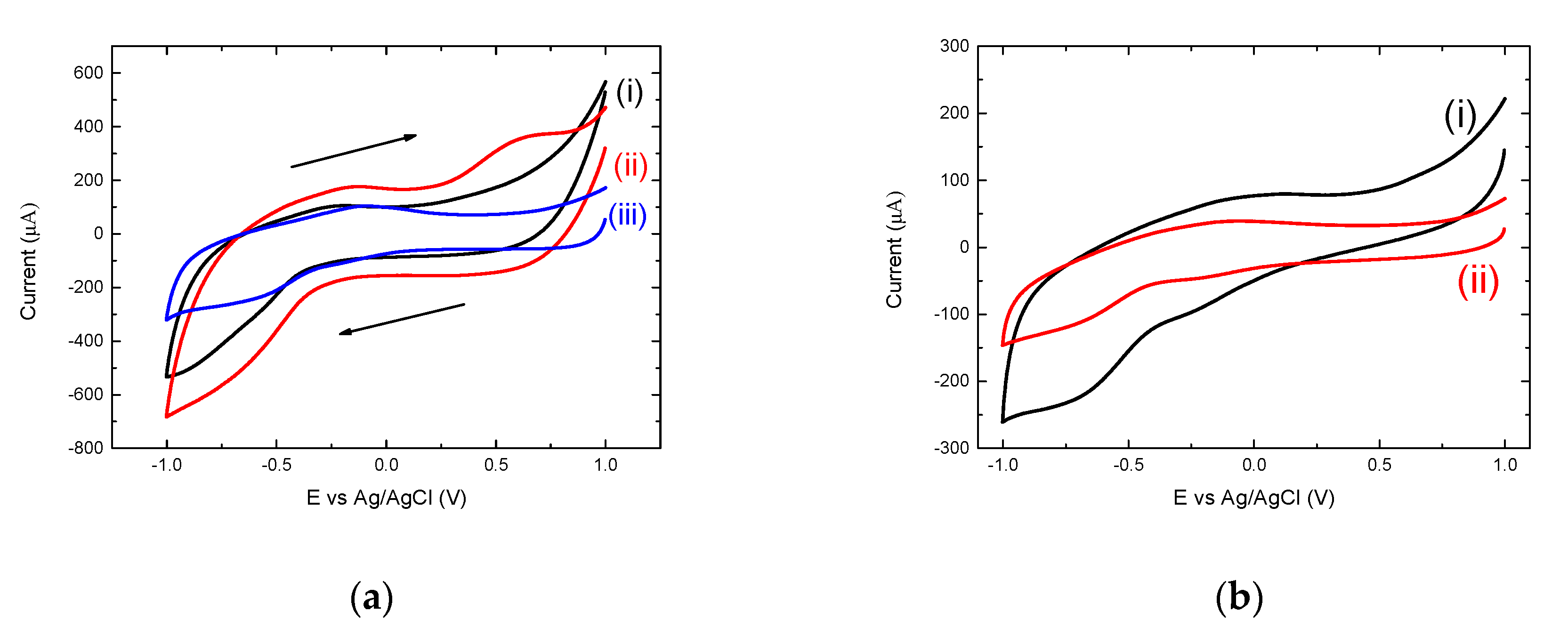
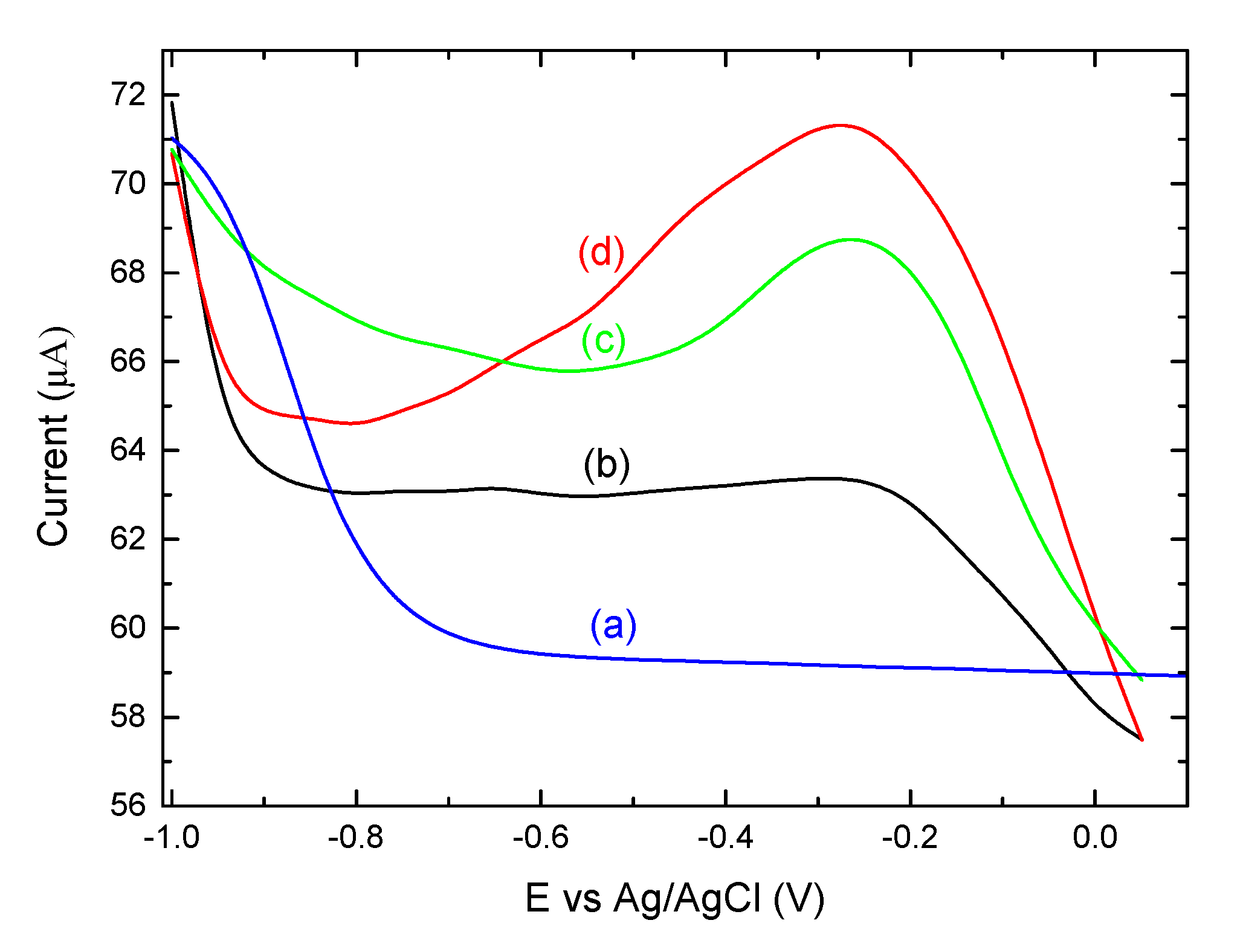
3.3. Electrochemical Analysis Showing Conjugation of (KG)5-MOG35-55 to OM
3.4. Complete Conjugation between (KG)5-MOG35-55 Peptide to OM is Monitored by HPLC
3.5. The Importance of the Linker (KG)5 for Conjugation of Peptides to OM
3.6. Mannan-Peptide Conjugate
3.7. Chemistry of the Mannose Cleavage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nosrati, R.; Olad, A.; Maryami, F. The use of graphite/TiO2 nanocomposite additive for preparation of polyacrylic based visible-light induced antibacterial and self-cleaning coating. Res. Chem. Intermed. 2018, 44, 6219–6237. [Google Scholar] [CrossRef]
- Nikolaou, P.; Deskoulidis, E.; Topoglidis, E.; Kakoulidou, A.T.; Tsopelas, F. Application of chemometrics for detection and modeling of adulteration of fresh cow milk with reconstituted skim milk powder using voltammetric fingerpriting on a graphite/SiO2 hybrid electrode. Talanta 2020, 206, 120223. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, P.; Vareli, I.; Deskoulidis, E.; Matsoukas, J.; Vassilakopoulou, A.; Koutselas, I.; Topoglidis, E. Graphite/SiO2 film electrode modified with hybrid organic-inorganic perovskites: Synthesis, optical, electrochemical properties and application in electrochemical sensing of losartan. J. Solid State Chem. 2019, 273, 17–24. [Google Scholar] [CrossRef]
- Topoglidis, E.; Kolozoff, P.-A.; Tiflidis, C.; Papavasiliou, J.; Sakellis, E. Adsorption and electrochemical behavior of Cyt-c on carbon nanotubes/TiO2 nanocomposite films fabricated at various annealing temperatures. Colloid Polym. Sci. 2018, 296, 1353–1364. [Google Scholar] [CrossRef]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; De Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple sclerosis: Immunopathology and treatment update. Brain Sci. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Katsara, M.; Apostolopoulos, V. Editorial: Multiple Sclerosis: Pathogenesis and Therapeutics. Med. Chem. 2018, 14, 104–105. [Google Scholar] [CrossRef]
- Katsara, M.; Matsoukas, J.; Deraos, G.; Apostolopoulos, V. Towards immunotherapeutic drugs and vaccines against multiple sclerosis. Acta Biochim. Biophys. Sin. 2008, 40, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Katsara, M.; Tselios, T.; Deraos, S.; Deraos, G.; Matsoukas, M.T.; Lazoura, E.; Matsoukas, J.; Apostolopoulos, V. Round and round we go: Cyclic peptides in disease. Curr. Med. Chem. 2006, 13, 2221–2232. [Google Scholar] [CrossRef]
- Steinman, L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell 1996, 85, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Steinman, L. Multiple sclerosis: A two-stage disease. Nat. Immunol. 2001, 2, 762–764. [Google Scholar] [CrossRef]
- Candia, M.; Kratzer, B.; Pickl, W.F. On Peptides and Altered Peptide Ligands: From Origin, Mode of Action and Design to Clinical Application (Immunotherapy). Int. Arch. Allergy Immunol. 2016, 170, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Minigo, G.; Plebanski, M.; Apostolopoulos, V. The good, the bad and the ugly: How altered peptide ligands modulate immunity. Expert Opin. Biol. Ther. 2008, 8, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. A double mutation of MBP(83-99) peptide induces IL-4 responses and antagonizes IFN-gamma responses. J. Neuroimmunol. 2008, 200, 77–89. [Google Scholar] [CrossRef]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Mannosylation of mutated MBP83-99 peptides diverts immune responses from Th1 to Th2. Mol. Immunol. 2008, 45, 3661–3670. [Google Scholar] [CrossRef]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Tselios, T.; Deraos, G.; Lourbopoulos, A.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Altered peptide ligands of myelin basic protein (MBP87-99) conjugated to reduced mannan modulate immune responses in mice. Immunology 2009, 128, 521–533. [Google Scholar] [CrossRef]
- Trager, N.N.M.; Butler, J.T.; Harmon, J.; Mount, J.; Podbielska, M.; Haque, A.; Banik, N.L.; Beeson, C.C. A Novel Aza-MBP Altered Peptide Ligand for the Treatment of Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2018, 55, 267–275. [Google Scholar] [CrossRef]
- Katsara, M.; Deraos, S.; Tselios, T.V.; Pietersz, G.; Matsoukas, J.; Apostolopoulos, V. Immune responses of linear and cyclic PLP139-151 mutant peptides in SJL/J mice: Peptides in their free state versus mannan conjugation. Immunotherapy 2014, 6, 709–724. [Google Scholar] [CrossRef]
- Lourbopoulos, A.; Deraos, G.; Matsoukas, M.T.; Touloumi, O.; Giannakopoulou, A.; Kalbacher, H.; Grigoriadis, N.; Apostolopoulos, V.; Matsoukas, J. Cyclic MOG35-55 ameliorates clinical and neuropathological features of experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2017, 25, 4163–4174. [Google Scholar] [CrossRef] [Green Version]
- Lourbopoulos, A.; Matsoukas, M.T.; Katsara, M.; Deraos, G.; Giannakopoulou, A.; Lagoudaki, R.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Cyclization of PLP139-151 peptide reduces its encephalitogenic potential in experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2018, 26, 2221–2228. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Rostami, A.; Matsoukas, J. The Long Road of Immunotherapeutics against Multiple Sclerosis. Brain Sci. 2020, 10. [Google Scholar] [CrossRef]
- Day, S.; Tselios, T.; Androutsou, M.E.; Tapeinou, A.; Frilligou, I.; Stojanovska, L.; Matsoukas, J.; Apostolopoulos, V. Mannosylated Linear and Cyclic Single Amino Acid Mutant Peptides Using a Small 10 Amino Acid Linker Constitute Promising Candidates Against Multiple Sclerosis. Front. Immunol. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Rodi, M.; Kalbacher, H.; Chatzantoni, K.; Karagiannis, F.; Synodinos, L.; Plotas, P.; Papalois, A.; Dimisianos, N.; Papathanasopoulos, P.; et al. Properties of myelin altered peptide ligand cyclo(87-99)(Ala91,Ala96)MBP87-99 render it a promising drug lead for immunotherapy of multiple sclerosis. Eur. J. Med. Chem. 2015, 101, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tapeinou, A.; Androutsou, M.E.; Kyrtata, K.; Vlamis-Gardikas, A.; Apostolopoulos, V.; Matsoukas, J.; Tselios, T. Conjugation of a peptide to mannan and its confirmation by tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 2015, 485, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Tselios, T.V.; Lamari, F.N.; Karathanasopoulou, I.; Katsara, M.; Apostolopoulos, V.; Pietersz, G.A.; Matsoukas, J.M.; Karamanos, N.K. Synthesis and study of the electrophoretic behavior of mannan conjugates with cyclic peptide analogue of myelin basic protein using lysine-glycine linker. Anal. Biochem. 2005, 347, 121–128. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Drakaki, H.; Loveland, B.E.; Piddlesden, S.J.; Plebanski, M.; Pouniotis, D.S.; Alexis, M.N.; et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835]. Breast Cancer Res. 2006, 8, R27. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Stojanovska, L.; McKenzie, I.F.; Vassilaros, S. Dendritic cell immunotherapy: Clinical outcomes. Clin. Transl. Immunol. 2014, 3, e21. [Google Scholar] [CrossRef] [Green Version]
- Karanikas, V.; Hwang, L.A.; Pearson, J.; Ong, C.S.; Apostolopoulos, V.; Vaughan, H.; Xing, P.X.; Jamieson, G.; Pietersz, G.; Tait, B.; et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J. Clin. Investig. 1997, 100, 2783–2792. [Google Scholar] [CrossRef] [Green Version]
- Karanikas, V.; Lodding, J.; Maino, V.C.; McKenzie, I.F. Flow cytometric measurement of intracellular cytokines detects immune responses in MUC1 immunotherapy. Clin. Cancer Res. 2000, 6, 829–837. [Google Scholar]
- Karanikas, V.; Thynne, G.; Mitchell, P.; Ong, C.S.; Gunawardana, D.; Blum, R.; Pearson, J.; Lodding, J.; Pietersz, G.; Broadbent, R.; et al. Mannan Mucin-1 Peptide Immunization: Influence of Cyclophosphamide and the Route of Injection. J. Immunother 2001, 24, 172–183. [Google Scholar] [CrossRef]
- Loveland, B.E.; Zhao, A.; White, S.; Gan, H.; Hamilton, K.; Xing, P.X.; Pietersz, G.A.; Apostolopoulos, V.; Vaughan, H.; Karanikas, V.; et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: A phase I trial in patients with adenocarcinoma. Clin. Cancer Res. 2006, 12, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.L.; Quinn, M.A.; Grant, P.T.; Allen, D.G.; Jobling, T.W.; White, S.C.; Zhao, A.; Karanikas, V.; Vaughan, H.; Pietersz, G.; et al. A phase 2, single-arm study of an autologous dendritic cell treatment against mucin 1 in patients with advanced epithelial ovarian cancer. J. Immunother Cancer 2014, 2, 16. [Google Scholar] [CrossRef]
- Vassilaros, S.; Tsibanis, A.; Tsikkinis, A.; Pietersz, G.A.; McKenzie, I.F.; Apostolopoulos, V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy 2013, 5, 1177–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deraos, G.; Chatzantoni, K.; Matsoukas, M.T.; Tselios, T.; Deraos, S.; Katsara, M.; Papathanasopoulos, P.; Vynios, D.; Apostolopoulos, V.; Mouzaki, A.; et al. Citrullination of linear and cyclic altered peptide ligands from myelin basic protein (MBP(87-99)) epitope elicits a Th1 polarized response by T cells isolated from multiple sclerosis patients: Implications in triggering disease. J. Med. Chem. 2008, 51, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87-99 cyclic analogue: Enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tselios, T.; Apostolopoulos, V.; Daliani, I.; Deraos, S.; Grdadolnik, S.; Mavromoustakos, T.; Melachrinou, M.; Thymianou, S.; Probert, L.; Mouzaki, A.; et al. Antagonistic effects of human cyclic MBP(87-99) altered peptide ligands in experimental allergic encephalomyelitis and human T-cell proliferation. J. Med. Chem. 2002, 45, 275–283. [Google Scholar] [CrossRef]
- Song, M.-J.; Hwang, S.W.; Whang, D. Amperometric hydrogen peroxide biosensor based on a modified gold electrode with silver nanowires. J. Appl. Electrochem. 2010, 40, 2099–2105. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri-and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Hussain, G.; Silvester, D.S. Comparison of Voltammetric Techniques for Ammonia Sensing in Ionic Liquids. Electroanalysis 2018, 30, 75–83. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Gordon, S.; Martinez-Pomares, L.; McKenzie, I.F. Aldehyde-mannan antigen complexes target the MHC class I antigen-presentation pathway. Eur. J. Immunol. 2000, 30, 1714–1723. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Loveland, B.E.; Sandrin, M.S.; McKenzie, I.F. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc. Natl. Acad. Sci. USA 1995, 92, 10128–10132. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, J.; Rejtar, T.; Sawant, R.; Wang, Z.; Torchilin, V.P. TAT peptide and its conjugates: Proteolytic stability. Bioconjug. Chem. 2009, 20, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Lemus, R.; Karol, M.H. Conjugation of haptens. Methods Mol. Med. 2008, 138, 167–182. [Google Scholar] [CrossRef]
- Berthet, M.; Martinez, J.; Parrot, I. MgI2 -chemoselective cleavage for removal of amino acid protecting groups: A fresh vision for peptide synthesis. Biopolymers 2017, 108. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Amino acid-protecting groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [Green Version]
- Apostolopoulos, V.; Deraos, G.; Matsoukas, M.T.; Day, S.; Stojanovska, L.; Tselios, T.; Androutsou, M.E.; Matsoukas, J. Cyclic citrullinated MBP87-99 peptide stimulates T cell responses: Implications in triggering disease. Bioorg. Med. Chem. 2017, 25, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Pagba, C.V.; McCaslin, T.G.; Veglia, G.; Porcelli, F.; Yohannan, J.; Guo, Z.; McDaniel, M.; Barry, B.A. A tyrosine-tryptophan dyad and radical-based charge transfer in a ribonucleotide reductase-inspired maquette. Nat. Commun. 2015, 6, 10010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, J.; Zhao, L.; Zheng, L.; Gao, F.; Gao, L.; Liu, R.; Wang, Y.; Gao, X. Peptide–Au Cluster Probe: Precisely Detecting Epidermal Growth Factor Receptor of Three Tumor Cell Lines at a Single-Cell Level. ACS Omega 2017, 2, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapeinou, A. Design, synthesis and evaluation of analogues of myelin protein immunodominant epitopes implemented in multiple sclerosis. Eur. J. Med. Chem. 2017, 143, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Barnes, N.; Pietersz, G.A.; McKenzie, I.F. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine 2000, 18, 3174–3184. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; McKenzie, I.F. Cell-mediated immune responses to MUC1 fusion protein coupled to mannan. Vaccine 1996, 14, 930–938. [Google Scholar] [CrossRef]
- Sheng, K.C.; Kalkanidis, M.; Pouniotis, D.S.; Wright, M.D.; Pietersz, G.A.; Apostolopoulos, V. The adjuvanticity of a mannosylated antigen reveals TLR4 functionality essential for subset specialization and functional maturation of mouse dendritic cells. J. Immunol. 2008, 181, 2455–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, K.C.; Pouniotis, D.S.; Wright, M.D.; Tang, C.K.; Lazoura, E.; Pietersz, G.A.; Apostolopoulos, V. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology 2006, 118, 372–383. [Google Scholar] [CrossRef] [PubMed]


| Acronym | Specification |
|---|---|
| MOG35-55 | Myelin oligodendrocyte glycoprotein immunogenic epitope, region 35–55 |
| MOG37-55 | Myelin oligodendrocyte glycoprotein immunogenic epitope, region 37–55 |
| (KG)5-MOG35-55 | Peptide analogue MOG35-55 with (KG)5 at the N-terminus |
| OM-(KG)5-MOG35-55 | Oxidized mannan conjugated to (KG)5-MOG35-55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deskoulidis, E.; Petrouli, S.; Apostolopoulos, V.; Matsoukas, J.; Topoglidis, E. The Use of Electrochemical Voltammetric Techniques and High-Pressure Liquid Chromatography to Evaluate Conjugation Efficiency of Multiple Sclerosis Peptide-Carrier Conjugates. Brain Sci. 2020, 10, 577. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10090577
Deskoulidis E, Petrouli S, Apostolopoulos V, Matsoukas J, Topoglidis E. The Use of Electrochemical Voltammetric Techniques and High-Pressure Liquid Chromatography to Evaluate Conjugation Efficiency of Multiple Sclerosis Peptide-Carrier Conjugates. Brain Sciences. 2020; 10(9):577. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10090577
Chicago/Turabian StyleDeskoulidis, Efstathios, Sousana Petrouli, Vasso Apostolopoulos, John Matsoukas, and Emmanuel Topoglidis. 2020. "The Use of Electrochemical Voltammetric Techniques and High-Pressure Liquid Chromatography to Evaluate Conjugation Efficiency of Multiple Sclerosis Peptide-Carrier Conjugates" Brain Sciences 10, no. 9: 577. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10090577