Pain Perception in Disorder of Consciousness: A Scoping Review on Current Knowledge, Clinical Applications, and Future Perspective
Abstract
:1. Introduction
Objective
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search
2.3. Selection of Sources of Evidence
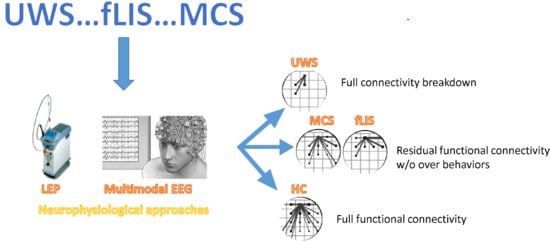
3. Results
3.1. Autonomic Nervous System
3.2. Laser-Evoked Potentials and Advanced EEG Signal Analyses
4. Discussion
4.1. Management Perspective
4.2. Future Research
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plum, F.; Posner, J.B. The diagnosis of stupor and coma. Contemp. Neurol. Ser. 1972, 10, 1–286. [Google Scholar] [PubMed]
- Royal College of Physicians. The vegetative state: Guidance on diagnosis and management. Clin. Med. 2003, 3, 249–254. [Google Scholar] [CrossRef]
- The Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters: Assessment and management of patients in the persistent vegetative state. Neurology 1995, 45, 1015–1018. [Google Scholar] [CrossRef] [Green Version]
- Laureys, S.; European Task Force on Disorders of Consciousness; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; Von Wild, K.R.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Schiff, N.D. Cognitive motor dissociation following severe brain injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef]
- Bekinschtein, T.; Niklison, J.; Sigman, L.; Manes, F.; Leiguarda, R.; Armony, J.; Owen, A.; Carpintiero, S.; Olmos, L. Emotion processing in the minimally conscious state. J. Neurol. Neurosurg. Psychiatry 2004, 75, 788. [Google Scholar] [CrossRef] [Green Version]
- Bekinschtein, T.A.; Manes, F.F.; Villarreal, M.; Owen, A.M.; Maggiore, V.D. Functional imaging reveals movement preparatory activity in the vegetative state. Front. Hum. Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Crone, J.S.; Bio, B.J.; Vespa, P.M.; Lutkenhoff, E.S.; Monti, M.M. Restoration of thalamo-cortical connectivity after brain injury: Recovery of consciousness, complex behavior, or passage of time? J. Neurosci. Res. 2017, 96, 671–687. [Google Scholar] [CrossRef] [Green Version]
- Duclos, C.; Dumont, M.; Arbour, C.; Paquet, J.; Blais, H.; Menon, D.K.; De Beaumont, L.; Bernard, F.; Gosselin, N. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology 2017, 88, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Laureys, S.; Faymonville, M.; Peigneuxad, P.; Damas, P.; Lambermont, B.; Del Fiore, G.; Degueldre, C.; Aerts, J.; Luxen, A.; Franck, G.; et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage 2002, 17, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.M. Cognition in the vegetative state. Annu. Rev. Clin. Psychol. 2012, 8, 431–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodien, Y.G.; Chatelle, C.; Edlow, B.L. Functional Networks in Disorders of Consciousness. Semin. Neurol. 2017, 37, 485–502. [Google Scholar]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Pickard, J.D. Detecting awareness in the vegetative state. Science 2006, 313, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riganello, F.; Cortese, M.D.; Dolce, G.; Sannita, W.G. Visual pursuit response in the severe disorder of consciousness: Modulation by the central autonomic system and a predictive model. BMC Neurol. 2013, 13, 164. [Google Scholar] [CrossRef] [Green Version]
- Riganello, F.; Cortese, M.D.; Dolce, G.; Lucca, L.F.; Sannita, W.G. The autonomic system functional state predicts responsiveness in DOC. J. Neurotrauma 2015, 32, 1071–1077. [Google Scholar] [CrossRef]
- Bruno, M.A.; Vanhaudenhuyse, A.; Thibaut, A.; Moonen, G.; Laureys, S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. J. Neurol. 2011, 258, 1373–1384. [Google Scholar] [CrossRef]
- Formisano, R.; D’Ippolito, M.; Catani, S. Functional locked-in syndrome as recovery phase of vegetative state. Brain Inj. 2013, 27, 1332. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Lotze, M. Instrumental methods in the diagnostics of locked-in syndrome. Restor. Neurol. Neurosci. 2013, 31, 25–40. [Google Scholar] [CrossRef]
- Fernández-Espejo, D.; Rossit, S.; Owen, A.M. A thalamocortical mechanism for the absence of overt motor behavior in covertly aware patients. JAMA Neurol. 2015, 72, 1442–1450. [Google Scholar] [CrossRef] [Green Version]
- Soddu, A.; Gómez, F.; Heine, L.; Di Perri, C.; Bahri, M.A.; Voss, H.U.; Bruno, M.; Vanhaudenhuyse, A.; Phillips, C.; Demertzi, A.; et al. Correlation between resting state fMRI total neuronal activity and PET metabolism in healthy controls and patients with disorders of consciousness. Brain Behav. 2015, 6, e00424. [Google Scholar] [CrossRef] [Green Version]
- Demertzi, A.; Antonopoulos, G.; Heine, L.; Voss, H.U.; Crone, J.S.; Angeles, C.D.L.; Bahri, M.A.; Di Perri, C.; Vanhaudenhuyse, A.; Charland-Verville, V.; et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 2015, 138, 2619–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Perri, C.; Bahri, M.A.; Amico, E.; Thibaut, A.; Heine, L.; Antonopoulos, G.; Charland-Verville, V.; Wannez, S.; Gomez, F.; Hustinx, R.; et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: A cross-sectional multimodal imaging study. Lancet Neurol. 2016, 15, 830–842. [Google Scholar] [CrossRef]
- Di Perri, C.; Bastianello, S.; Bartsch, A.J.; Pistarini, C.; Maggioni, G.; Magrassi, L.; Imberti, R.; Pichiecchio, A.; Vitali, P.; Laureys, S.; et al. Limbic hyperconnectivity in the vegetative state. Neurology 2013, 81, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Laureys, S.; Faymonville, M.-E.; Luxen, A.; Lamy, M.; Franck, G.; Maquet, P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 2000, 355, 1790–1791. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.M.; Rosenberg, M.; Finoia, P.; Kamau, E.; Pickard, J.D.; Owen, A.M. Thalamo-frontal connectivity mediates top-down cognitive functions in disorders of consciousness. Neurology 2014, 84, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK coma recovery scale-revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef]
- Chatelle, C.; De Val, M.D.; Catano, A.; Chaskis, C.; Seeldrayers, P.; Laureys, S.; Biston, P.; Schnakers, C. Is the Nociception Coma Scale-Revised a useful clinical tool for managing pain in patients with disorders of consciousness? Clin. J. Pain 2016, 32, 321–326. [Google Scholar]
- Chatelle, C.; Majerus, S.; Whyte, J.; Laureys, S.; Schnakers, C. A sensitive scale to assess nociceptive pain in patients with disorders of consciousness. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1233–1237. [Google Scholar] [CrossRef] [Green Version]
- Chatelle, C.; Hauger, S.L.; Martial, C.; Becker, F.; Eifert, B.; Boering, D.; Giacino, J.T.; Laureys, S.; Løvstad, M.; Maurer-Karattup, P. Assessment of Nociception and Pain in Participants in an Unresponsive or Minimally Conscious State after Acquired Brain Injury: The Relation Between the Coma Recovery Scale-Revised and the Nociception Coma Scale-Revised. Arch. Phys. Med. Rehabil. 2018, 99, 1755–1762. [Google Scholar] [PubMed] [Green Version]
- Coghill, R.C.; McHaffie, J.G.; Yen, Y.-F. Neural correlates of interindividual differences in the subjective experience of pain. Proc. Natl. Acad. Sci. USA 2003, 100, 8538–8542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnakers, C.; Chatelle, C.; Vanhaudenhuyse, A.; Majerus, S.; LeDoux, D.; Boly, M.; Bruno, M.-A.; Boveroux, P.; Demertzi, A.; Moonen, G.; et al. The Nociception Coma Scale: A new tool to assess nociception in disorders of consciousness. Pain 2010, 148, 215–219. [Google Scholar] [CrossRef]
- Schnakers, C.; Zasler, N.D. Pain assessment and management in disorders of consciousness. Curr. Opin. Neurol. 2007, 20, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Riganello, F.; Arcuri, F.C.; Candelieri, A.; Guglielmino, F.; Dolce, G.; Sannita, W.; Schnakers, C. A study of the reliability of the Nociception Coma Scale. Clin. Rehabil. 2014, 29, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Simini, B. Patients’ perceptions of intensive care. Lancet 1999, 354, 571–572. [Google Scholar] [CrossRef]
- Boly, M.; Faymonville, M.-E.; Schnakers, C.; Peigneux, P.; Lambermont, B.; Phillips, C.; Lancellotti, P.; Luxen, A.; Lamy, M.; Moonen, G.; et al. Perception of pain in the minimally conscious state with PET activation: An observational study. Lancet Neurol. 2008, 7, 1013–1020. [Google Scholar] [CrossRef]
- Celesia, G.G.; Sannita, W.G. Can patients in vegetative state experience pain and have conscious awareness? Neurology 2013, 80, 328–329. [Google Scholar] [CrossRef] [Green Version]
- Markl, A.; Yu, T.; Vogel, D.; Muller, F.; Kotchoubey, B.; Lang, S. Brain processing of pain in patients with unresponsive wakefulness syndrome. Brain Behav. 2013, 3, 95–103. [Google Scholar] [CrossRef]
- Yu, T.; Lang, S.; Vogel, D.; Markl, A.; Mueller, F.; Kotchoubey, B. Patients with unresponsive wakefulness syndrome respond to the pain cries of other people. Neurology 2013, 80, 345–352. [Google Scholar] [CrossRef]
- Graham, M. Can they Feel? The Capacity for Pain and Pleasure in Patients with Cognitive Motor Dissociation. Neuroethics 2019, 12, 153–169. [Google Scholar] [CrossRef] [Green Version]
- Kassubek, J.; Juengling, F.D.; Els, T.; Spreer, J.; Herpers, M.; Krause, T.; Moser, E.; Lücking, C.H. Activation of a residual cortical network during painful stimulation in long-term postanoxic vegetative state: A OPET study. J. Neurol. Sci. 2003, 212, 85–91. [Google Scholar] [CrossRef]
- Schnakers, C.; Chatelle, C.; Majerus, S.; Gosseries, O.; de Val, M.; Laureys, S. Assessment and detection of pain in noncommunicative severely brain-injured patients. Expert Rev. Neurother. 2010, 10, 1725–1731. [Google Scholar] [CrossRef] [Green Version]
- Zanatta, P.; Benvenuti, S.M.; Baldanzi, F.; Bendini, M.; Saccavini, M.; Tamari, W.; Palomba, D.; Bosco, E. Pain-related somatosensory evoked potentials and functional brain magnetic resonance in the evaluation of neurologic recovery after cardiac arrest: A case study of three patients. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 22. [Google Scholar] [CrossRef] [Green Version]
- Laureys, S. The neural correlate of (un) awareness: Lessons from the vegetative state. Trends Cogn. Sci. 2005, 9, 556–559. [Google Scholar] [CrossRef]
- Giacino, J.T.; Hirsch, J.; Schiff, N.; Laureys, S. Functional neuroimaging applications for assessment and rehabilitation planning in patients with disorders of consciousness. Arch. Phys. Med. Rehabil. 2006, 87, S67–S76. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, F.; Sacco, S.; Stewart, J.; Sarà, M.; Carolei, A. Disorders of Consciousness: Painless or Painful Conditions?—Evidence from Neuroimaging Studies. Brain Sci. 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnakers, C.; Chatelle, C.; Demertzi, A.; Majerus, S.; Laureys, S. What about pain in disorders of consciousness? AAPS J. 2012, 14, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Chatelle, C.; Thibaut, A.; Whyte, J.; De Val, M.D.; Laureys, S.; Schnakers, C. Pain issues in disorders of consciousness. Brain Inj. 2014, 28, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Riganello, F.; Soddu, A.; Tonin, P. Addressing Pain for a Proper Rehabilitation Process in Patients with Severe Disorders of Consciousness. Front. Pharmacol. 2021, 12, 628980. [Google Scholar]
- Dolce, G.; Lucca, L.F. The vegetative state updated. J. Psychophysiol. 2010, 24, 107–111. [Google Scholar] [CrossRef]
- Peterson, A.; Cruse, D.; Naci, L.; Weijer, C.; Owen, A.M. Risk, diagnostic error, and the clinical science of consciousness. NeuroImage Clin. 2015, 7, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A. Consilience, clinical validation, and global disorders of consciousness. Neurosci. Conscious. 2016, 2016, niw011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauger, S.L.; Schanke, A.K.; Andersson, S.; Chatelle, C.; Schnakers, C.; Løvstad, M. The Clinical Diagnostic Utility of Electrophysiological Techniques in Assessment of Patients with Disorders of Consciousness Following Acquired Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2017, 32, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [Green Version]
- ASP Taxonomy Working Group. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. (1986). Pain. Suppl. 1986, 3, S1–S226. [Google Scholar]
- American Congress of Rehabilitation Medicine, Brain Injury-Interdisciplinary Special Interest Group, Disorders of Consciousness Task Force; Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.; et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef]
- Harrington, M.H. Advances in Neuroimaging and the Vegetative State: Implications for End-of-Life Care. Hamline Law Rev. 2013, 36, 213. [Google Scholar]
- Riganello, F.; Macrì, S.; Alleva, E.; Petrini, C.; Soddu, A.; Leòn-Carriòn, J.; Dolce, G. Pain Perception in Unresponsive Wakefulness Syndrome May Challenge the Interruption of Artificial Nutrition and Hydration: Neuroethics in Action. Front. Neurol. 2016, 7, 202. [Google Scholar] [CrossRef] [Green Version]
- Leo, A.; Naro, A.; Cannavò, A.; Pisani, L.R.; Bruno, R.; Salviera, C.; Bramanti, P.; Calabrò, R. Could autonomic system assessment be helpful in disorders of consciousness diagnosis? A neurophysiological study. Exp. Brain Res. 2016, 234, 2189–2199. [Google Scholar] [CrossRef]
- Riganello, F.; Larroque, S.K.; Di Perri, C.; Prada, V.; Sannita, W.G.; Laureys, S. Measures of CNS-Autonomic Interaction and Responsiveness in Disorder of Consciousness. Front. Neurosci. 2019, 13, 530. [Google Scholar] [CrossRef]
- Friedman, B.H. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F. Responsiveness and the autonomic control-CNS two-way interaction in disorders of consciousness. In Brain Function and Responsiveness in Disorders of Consciousness; Monti, M.M., Sannita, W.G., Eds.; Springer: Cham, Switzerland, 2016; pp. 145–155. [Google Scholar]
- Berntson, G.G.; Cacioppo, J.T. Heart rate variability: Stress and psychiatric conditions. In Dynamic Electrocardiography; Malik, M., Camm, A.J., Eds.; Blackwell/Futura: New York, NY, USA, 2004; pp. 57–64. [Google Scholar]
- Hagemann, D.; Waldstein, S.R.; Thayer, J.F. Central and autonomic nervous system integration in emotion. Brain Cogn. 2003, 52, 79–87. [Google Scholar] [CrossRef]
- Riganello, F.; Dolce, G.; Sannita, W. Heart rate variability and the central autonomic network in the severe disorder of consciousness. J. Rehabil. Med. 2012, 44, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Thayer, J.F.; Sternberg, E. Beyond heart rate variability. Ann. N. Y. Acad. Sci. 2006, 1088, 361–372. [Google Scholar] [CrossRef]
- Devalle, G.; Castiglioni, P.; Arienti, C.; Abbate, C.; Mazzucchi, A.; Agnello, L.; Merati, G. Cardio-respiratory autonomic responses to nociceptive stimuli in patients with disorders of consciousness. PLoS ONE 2018, 13, e0201921. [Google Scholar] [CrossRef]
- Riganello, F.; Chatelle, C.; Schnakers, C.; Laureys, S. Heart Rate Variability as an Indicator of Nociceptive Pain in Disorders of Consciousness? J. Pain Symptom Manag. 2019, 57, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Turkstra, L.Y.N.S. Brain injury. Brain Inj. 1995, 9, 61–80. [Google Scholar] [CrossRef]
- Luauté, J.; Dubois, A.; Heine, L.; Guironnet, C.; Juliat, A.; Gaveau, V.; Tillmann, B.; Perrin, F. Electrodermal reactivity to emotional stimuli in healthy subjects and patients with disorders of consciousness. Ann. Phys. Rehabil. Med. 2018, 61, 401–406. [Google Scholar] [CrossRef]
- Hildebrandt, H.; Zieger, A.; Engel, A.; Fritz, K.W.; Bussmann, B. Differentiation of autonomic nervous activity in different stages of coma displayed by power spectrum analysis of heart rate variability. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 46–52. [Google Scholar] [CrossRef]
- Keller, I.; Hülsdunk, A.; Müller, F. The influence of acoustic and tactile stimulation on vegetative parameters and EEG in persistent vegetative state. Funct. Neurol. 2007, 22, 159–163. [Google Scholar]
- De Tommaso, M.; Navarro, J.; Ricci, K.; Lorenzo, M.; Lanzillotti, C.; Colonna, F.; Resta, M.; Lancioni, G.; Livrea, P. Pain in prolonged disorders of consciousness: Laser evoked potentials findings in patients with vegetative and minimally conscious states. Brain Inj. 2013, 27, 962–972. [Google Scholar] [CrossRef]
- De Tommaso, M.; Navarro, J.; Lanzillotti, C.; Ricci, K.; Buonocunto, F.; Livrea, P.; Lancioni, G.E. Cortical responses to salient nociceptive and not nociceptive stimuli in vegetative and minimal conscious state. Front. Hum. Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturella, I.; Crivelli, D.; Fossati, M.; Fiorillo, F.; Balconi, M. EEG and autonomic responses to nociceptive stimulation in disorders of consciousness. J. Clin. Neurosci. 2019, 60, 101–106. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, S.; Naro, A.; Bonanno, L.; Russo, M.; Muscarà, N.; Bramanti, P.; Marino, S. Assessment of nociceptive system in vegetative and minimally conscious state by using laser evoked potentials. Brain Inj. 2015, 29, 1467–1474. [Google Scholar] [CrossRef]
- Naro, A.; Russo, M.; Leo, A.; Rifici, C.; Pollicino, P.; Bramanti, P.; Calabrò, R. Cortical Responsiveness to Nociceptive Stimuli in Patients with Chronic Disorders of Consciousness: Do C-Fiber Laser Evoked Potentials Have a Role? PLoS ONE 2015, 10, e0144713. [Google Scholar]
- Naro, A.; Leo, A.; Russo, M.; Quartarone, A.; Bramanti, P.; Calabrò, R.S. Shaping thalamo-cortical plasticity: A marker of cortical pain integration in patients with post-anoxic unresponsive wakefulness syndrome? Brain Stimul. 2015, 8, 97–104. [Google Scholar] [CrossRef]
- Naro, A.; Leo, A.; Cannavò, A.; Buda, A.; Bramanti, P.; Calabrò, R.S. Do unresponsive wakefulness syndrome patients feel pain? Role of laser-evoked potential-induced gamma-band oscillations in detecting cortical pain processing. Neuroscience 2016, 317, 141–148. [Google Scholar] [CrossRef]
- Aricò, I.; Naro, A.; Pisani, L.R.; Leo, A.; Muscarà, N.; De Salvo, S.; Silvestri, R.; Bramanti, P.; Calabrò, R.S. Could combined sleep and pain evaluation be useful in the diagnosis of disorders of consciousness (DOC)? Preliminary findings. Brain Inj. 2016, 30, 159–163. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, P.; Bramanti, A.; Calabrò, R.S. Assessing pain in patients with chronic disorders of consciousness: Are we heading in the right direction? Conscious. Cogn. 2017, 55, 148–155. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Manuli, A.; Leo, A.; De Luca, R.; Lo Buono, V.; Russo, M.; Bramanti, A.; Bramanti, P. Pain perception in patients with chronic disorders of consciousness: What can limbic system tell us? Clin. Neurophysiol. 2017, 128, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Leo, A.; Bramanti, P.; Calabrò, R.S. Moving Toward Conscious Pain Processing Detection in Chronic Disorders of Consciousness: Anterior Cingulate Cortex Neuromodulation. J. Pain 2015, 16, 1022–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naro, A.; Calabrò, R.S.; Pollicino, P.; Lombardo, C.; Bramanti, P. Unexpected recovery from a vegetative state or misdiagnosis? Lesson learned from a case report. NeuroRehabilitation 2017, 41, 735–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018, 91, 450–460. [Google Scholar]
- Dolce, G.; Riganello, F.; Quintieri, M.; Candelieri, A.; Conforti, D. Personal interaction in the vegetative state: A data-mining study. J. Psychophysiol. 2008, 22, 150–156. [Google Scholar] [CrossRef]
- Gutierrez, J.; Machado, C.; Estévez, M.; Olivares, A.; Hernández, H.; Perez, J.; Beltran, C.; Leisman, G. Heart rate variability changes induced by auditory stimulation in persistent vegetative state. Int. J. Disabil. Hum. Dev. 2010, 9, 357–362. [Google Scholar] [CrossRef]
- Laureys, S.; Perrin, F.; Brédart, S. Self-consciousness in non-communicative patients. Conscious Cogn. 2007, 16, 722–741. [Google Scholar] [CrossRef]
- Span-Sluyter, C.A.M.F.H.; Lavrijsen, J.C.M.; Van Leeuwen, E.; Koopmans, R.T.C.M. Moral dilemmas and conflicts concerning patients in a vegetative state/unresponsive wakefulness syndrome: Shared or non-shared decision making? A qualitative study of the professional perspective in two moral case deliberations. BMC Med. Ethics 2018, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riganello, F.; Candelieri, A.; Dolce, G.; Sannita, W.G. Residual emotional processing in the vegetative state: A scientific issue? Clin. Neurophysiol. 2011, 122, 1061–1062. [Google Scholar] [CrossRef]
- Andrews, K.; Murphy, L.; Munday, R.; Littlewood, C. Misdiagnosis of the vegetative state: Retrospective study in a rehabilitation unit. BMJ 1996, 313, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, A.; Lancioni, G.E.; Belardinelli, M.O.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J. Vegetative state: Efforts to curb misdiagnosis. Cogn. Process 2010, 11, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Gill-Thwaites, H. Lotteries, loopholes and luck: Misdiagnosis in the vegetative state patient. Brain Inj. 2006, 20, 1321–1328. [Google Scholar] [CrossRef]
- Laureys, S. Death, unconsciousness and the brain. Nat. Rev. Neurosci. 2005, 6, 899–909. [Google Scholar] [CrossRef]
- Demertzi, A.; Schnakers, C.; LeDoux, D.; Chatelle, C.; Bruno, M.-A.; Vanhaudenhuyse, A.; Boly, M.; Moonen, G.; Laureys, S. Different beliefs about pain perception in the vegetative and minimally conscious states: A European survey of medical and paramedical professionals. Prog. Brain Res. 2009, 177, 329–338. [Google Scholar]
- Cruse, D.; Owen, A.M. Consciousness revealed: New insights into the vegetative and minimally conscious states. Curr. Opin. Neurol. 2010, 23, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Balconi, M.; Arangio, R. The relationship between coma near coma, disability ratings, and event-related potentials in patients with disorders of consciousness: A semantic association task. Appl. Psychophysiol. Biofeedback 2015, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Arangio, R.; Guarnerio, C. Consciousness and N400 ERP measures in response to a semantic task. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 237–243. [Google Scholar] [CrossRef]
- Boly, M.; Faymonville, M.-E.; Peigneux, P.; Lambermont, B.; Damas, F.; Luxen, A.; Lamy, M.; Moonen, G.; Maquet, P.; Laureys, S. Cerebral processing of auditory and noxious stimuli in severely brain injured patients: Differences between VS and MCS. Neuropsychol. Rehabil. 2005, 15, 283–289. [Google Scholar] [CrossRef]
- Garbarino, S.; Sannita, W.G. DoC: A pathophysiological continuum with high variabiity? Neurology 2015. [Google Scholar] [CrossRef]
- King, D.R.; Ogilvie, M.P.; Pereira, B.M.; Chang, Y.; Manning, R.J.; Conner, J.A.; Schulman, C.I.; McKenney, M.G.; Proctor, K.G. Heart rate variability as a triage tool in patients with trauma during prehospital helicopter transport. J. Trauma 2009, 67, 436–440. [Google Scholar] [PubMed]
- Ryan, M.L.; Thorson, C.M.; Otero, C.A.; Vu, T.; Proctor, K.G. Clinical applications of heart rate variability in the triage and assessment of traumatically injured patients. Anesthesiol. Res. Pract. 2011, 2011, e416590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sannita, W.G. Responsiveness in DoC and individual variability. Front. Hum. Neurosci. 2015, 9, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijnen, V.J.; Heutink, M.; van Boxtel, G.J.; Eilander, H.J.; de Gelder, B. Autonomic reactivity to sensory stimulation is related to consciousness level after severe traumatic brain injury. Clin. Neurophysiol. 2006, 117, 1794–1807. [Google Scholar] [CrossRef]
- Abbate, C.; Trimarchi, P.D.; Basile, I.; Mazzucchi, A.; Devalle, G. Sensory stimulation for patients with disorders of consciousness: From stimulation to rehabilitation. Front. Hum. Neurosci. 2014, 8, 616. [Google Scholar]
- Bekinschtein, T.A.; Golombek, D.A.; Simonetta, S.H.; Coleman, M.R.; Manes, F.F. Circadian rhythms in the vegetative state. Brain Inj. 2009, 23, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Lechinger, J.; Santhi, N.; Del Giudice, R.; Gnjezda, M.-T.; Pichler, G.; Scarpatetti, M.; Donis, J.; Michitsch, G.; Schabus, M. Significance of circadian rhythms in severely brain-injured patients: A clue to consciousness? Neurology 2017, 88, 1933–1941. [Google Scholar]
- Candelieri, A.; Cortese, M.D.; Dolce, G.; Riganello, F.; Sannita, W.G. Visual pursuit: Within-day variability in the severe disorder of consciousness. J. Neurotrauma 2011, 28, 2013–2017. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Lang, S.; Mezger, G.; Schmalohr, D.; Schneck, M.; Semmler, A.; Bostanov, V.; Birbaumer, N. Information processing in severe disorders of consciousness: Vegetative state and minimally conscious state. Clin. Neurophysiol. 2005, 116, 2441–2453. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, J.H.; Kim, A.R.; Park, M.; Kim, T.-W. Neurobehavioral recovery in patients who emerged from prolonged disorder of consciousness: A retrospective study. BMC Neurol. 2020, 20, 198. [Google Scholar] [CrossRef]
- Giacino, J.T. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Owen, A.M.; Schiff, N.D. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004, 3, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Jolles, D.; Pickard, J.D. Response to comments on “Detecting awareness in the vegetative state”. Science 2007, 315, 1221. [Google Scholar] [CrossRef] [Green Version]
- Stender, J.; Gosseries, O.; Bruno, M.A.; Charland-Verville, V.; Vanhaudenhuyse, A.; Demertzi, A.; Chatelle, C.; Thonnard, M.; Thibaut, A.; Heine, L.; et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet 2014, 384, 514–522. [Google Scholar] [CrossRef]
- Enøe, C.; Georgiadis, M.P.; Johnson, W.O. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev. Vet. Med. 2000, 45, 61–81. [Google Scholar] [CrossRef]
- Cruse, D.; Chennu, S.; Chatelle, C.; Bekinschtein, T.A.; Fernández-Espejo, D.; Pickard, J.D.; Laureys, S.; Owen, O.M. Bedside detection of awareness in the vegetative state: A cohort study. Lancet 2011, 378, 2088–2094. [Google Scholar] [CrossRef] [Green Version]
- Goldfine, A.M.; Bardin, J.C.; Noirhomme, Q.; Fins, J.J.; Schiff, N.D.; Victor, J.D. Reanalysis of “Bedside detection of awareness in the vegetative state: A cohort study”. Lancet 2013, 381, 289–291. [Google Scholar] [CrossRef] [Green Version]
- Bayne, T.; Hohwy, J. Global disorders of consciousness. WIREs Cogn. Sci. 2014, 5, 129–138. [Google Scholar] [CrossRef]
- Shea, N.; Bayne, T. The vegetative state and the science of consciousness. Br. J. Philos. Sci. 2010, 61, 459–484. [Google Scholar] [CrossRef] [Green Version]
- Demertzi, A.; Racine, E.; Bruno, M.A.; Ledoux, D.; Gosseries, O.; Vanhaudenhuyse, A.; Thonnard, M.; Soddu, A.; Moonen, G.; Laureys, S. Pain perception in disorders of consciousness: Neuroscience, clinical care, and ethics in dialogue. Neuroethics 2013, 6, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Chatelle, C.; Thibaut, A.; Bruno, M.A.; Boly, M.; Bernard, C.; Hustinx, R.; Schnakers, C.; Laureys, S. Nociception coma scale-revised scores correlate with metabolism in the anterior cingulate cortex. Neurorehabilit. Neural Repair 2014, 28, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuillen, M.P. Can people who are unconscious or in the “vegetative state” perceive pain? Issues Law Med. 1991, 6, 373–383. [Google Scholar] [PubMed]
- Fossati, M.B.; Filippini, M.; Bonin, E.; Bornheim, S.; Lejeune, N.; Bodart, O.; Laureys, S.; Thibaut, A.; Chatelle, C. Pain management in disorders of consciousness. In Proceedings of the Frontier Neuroscience Conference Abstract: Belgian Brain Congress 2018—Belgian Brain Council, Liege, Belgium, 9–19 October 2018. [Google Scholar] [CrossRef]
- Chang, P.F.; Arendt-Nielsen, L.; Chen, A.C.N. Dynamic changes and spatial correlation of EEG activities during cold pressor test in man. Brain Res. Bull. 2002, 57, 667–675. [Google Scholar] [CrossRef]
- Goudman, L.; Laton, J.; Brouns, R.; Nagels, G.; Huysmans, E.; Buyl, R.; Ickmans, K.; Nijs, J.; Moens, M. Cortical mapping of painful electrical stimulation by quantitative electroencephalography: Unraveling the time-frequency-channel domain. J. Pain. Res. 2017, 10, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Shen, K.; Yu, K.; Wilder-Smith, E.P.V.; Li, X. Frequency-domain EEG source analysis for acute tonic cold pain perception. Clin. Neurophysiol. 2012, 123, 2042–2049. [Google Scholar] [CrossRef]
- Huber, M.T.; Bartling, J.; Pachur, D.; Woikowsky-Biedau, S.V.; Lautenbacher, S. EEG responses to tonic heat pain. Exp. Brain Res. 2006, 173, 14–24. [Google Scholar] [CrossRef]
- Dindo, L.; Fowles, D.C. The skin conductance orienting response to semantic stimuli: Significance can be independent of arousal. Psychophysiology 2008, 45, 111–118. [Google Scholar] [CrossRef]
- Sokolov, E.N. Higher nervous functions: The orienting reflex. Annu. Rev. Physiol. 1963, 25, 545–580. [Google Scholar] [CrossRef]
- Barry, R.J. Habituation of the orienting reflex and the development of Preliminary Process Theory. Neurobiol. Learn. Mem. 2009, 92, 235–242. [Google Scholar] [CrossRef]
- Bauer, R.M. Autonomic recognition of names and faces in prosopagnosia: A neuropsychological application of the guilty knowledge test. Neuropsychologia 1984, 22, 457–469. [Google Scholar] [CrossRef]
- Goldfine, A.M.; Victor, J.D.; Conte, M.M.; Bardin, J.C.; Schiff, N.D. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin. Neurophysiol. 2011, 122, 2157–2168. [Google Scholar] [CrossRef] [Green Version]
- Cruse, D.; Chennu, S.; Fernández-Espejo, D.; Payne, W.L.; Young, G.B.; Owen, A.M. Detecting awareness in the vegetative state: Electroencephalographic evidence for attempted movements to command. PLoS ONE 2012, 7, e49933. [Google Scholar] [CrossRef]
- Cruse, D.; Chennu, S.; Chatelle, C.; Fernández-Espejo, D.; Bekinschtein, T.A.; Pickard, J.D.; Laureys, S.; Owen, A.M. Relationship between etiology and covert cognition in the minimally conscious state. Neurology 2012, 78, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Lulé, D.; Noirhomme, Q.; Kleih, S.C.; Chatelle, C.; Halder, S.; Demertzi, A.; Bruno, M.-A.; Gosseries, A.; Vanhaudenhuyse, A.; Schnakers, C.; et al. Probing command following in patients with disorders of consciousness using a brain-computer interface. Clin. Neurophysiol. 2013, 124, 101–106. [Google Scholar] [CrossRef]
- Laureys, S.; Schiff, N.D. Coma and consciousness: Paradigms (re)framed by neuroimaging. NeuroImage 2012, 61, 478–491. [Google Scholar] [CrossRef] [Green Version]
- Billeri, L.; Filoni, S.; Russo, E.F.; Portaro, S.; Militi, D.; Calabrò, R.S.; Naro, A. Toward Improving Diagnostic Strategies in Chronic Disorders of Consciousness: An Overview on the (Re-)Emergent Role of Neurophysiology. Brain Sci. 2020, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Naro, A.; Calabrò, R.S.; Nagamine, T. Frontiers in Detecting Consciousness: The Growing Use of EEG Analysis. Innov. Clin. Neurosci. 2020, 17, 8–9. [Google Scholar]
- Tomaiuolo, F.; Cecchetti, L.; Gibson, R.M.; Logi, F.; Owen, A.M.; Malasoma, F.; Cozza, S.; Pietrini, P.; Ricciardi, E. Progression from Vegetative to Minimally Conscious State Is Associated with Changes in Brain Neural Response to Passive Tasks: A Longitudinal Single-Case Functional MRI Study. J. Int. Neuropsychol. Soc. 2016, 22, 620–630. [Google Scholar] [CrossRef]
- Pistoia, F.; Sacco, S.; Sarà, M.; Franceschini, M.; Carolei, A. Intrathecal baclofen: Effects on spasticity, pain, and consciousness in disorders of consciousness and locked-in syndrome. Curr. Pain Headache Rep. 2015, 19, 466. [Google Scholar] [CrossRef]
- Sarà, M.; Pistoia, F.; Mura, E.; Onorati, P.; Govoni, S. Intrathecal baclofen in patients with persistent vegetative state: 2 hypotheses. Arch. Phys. Med. Rehabil. 2009, 90, 1245–1249. [Google Scholar] [CrossRef]
- Pistoia, F.; Mura, E.; Govoni, S.; Fini, M.; Sarà, M. Awakenings and awareness recovery in disorders of consciousness: Is there a role for drugs? CNS Drugs 2010, 24, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, F.; Sarà, M.; Sacco, S.; Franceschini, M.; Carolei, A. Silencing the brain may be better than stimulating it. The GABA Effect. Curr. Pharm. Des. 2014, 20, 4154–4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, E.; Pistoia, F.; Sara, M.; Sacco, S.; Carolei, A.; Govoni, S. Pharmacological modulation of the state of awareness in patients with disorders of consciousness: An overview. Curr. Pharm. Des. 2014, 20, 4121–4139. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Katayama, Y.; Obuchi, T.; Kobayashi, K.; Oshima, H.; Fukaya, C. Deep brain stimulation and spinal cord stimulation for vegetative state and minimally conscious state. World Neurosurg. 2013, 80, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.; Fins, J.J.; Machado, A.; Schiff, N.D. Central thalamic deep brain stimulation to promote recovery from chronic posttraumatic minimally conscious state: Challenges and opportunities. Neuromodulation 2012, 15, 339–349. [Google Scholar] [CrossRef]
- Thibaut, A.; Bruno, M.A.; Ledoux, D.; Demertzi, A.; Laureys, S. TDCS in patients with disorders of consciousness: Sham-controlled randomized double-blind study. Neurology 2014, 82, 1112–1118. [Google Scholar] [CrossRef]
- Pistoia, F.; Sacco, S.; Carolei, A.; Sarà, M. Corticomotor facilitation in vegetative state: Results of a pilot study. Arch. Phys. Med. Rehabil. 2013, 94, 1599–1606. [Google Scholar] [CrossRef]
- Gosseries, O.; Pistoia, F.; Charland-Verville, V.; Carolei, A.; Sacco, S.; Laureys, S. The role of neuroimaging techniques in establishing diagnosis, prognosis and therapy in disorders of consciousness. Open Neuroimaging J. 2016, 10, 52–68. [Google Scholar] [CrossRef] [Green Version]
- Laureys, S.; Giacino, J.T.; Schiff, N.D.; Schabus, M.; Owen, A.M. How should functional imaging of patients with disorders of consciousness contribute to their clinical rehabilitation needs? Curr. Opin. Neurol. 2006, 19, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Noirhomme, Q.; Lesenfants, D.; Gomez, F.; Soddu, A.; Schrouff, J.; Garraux, G.; Luxen, A.; Phillips, C.; Laureys, S. Biased binomial assessment of cross-validated estimation of classification accuracies illustrated in diagnosis predictions. Neuroimage Clin. 2014, 4, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Cruse, D.; Beukema, S.; Chennu, S.; Malins, J.G.; Owen, A.M.; McRae, K. The reliability of the N400 in single subjects: Implications for patients with disorders of consciousness. Neuroimage Clin. 2014, 4, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Forgacs, P.B.; Conte, M.M.; Fridman, E.A.; Voss, H.U.; Victor, J.D.; Schiff, N.D. Preservation of Electroencephalographic Organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann. Neurol. 2014, 76, 869–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.M.; Fernández-Espejo, D.; Gonzalez-Lara, L.E.; Kwan, B.Y.; Lee, D.H.; Owen, A.M.; Cruse, D. Multiple tasks and neuroimaging modalities increase the likelihood of detecting covert awareness in patients with disorders of consciousness. Front. Hum. Neurosci. 2014, 8, 950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruse, D.; Gantner, I.; Soddu, A.; Owen, A.M. Lies, damned lies and diagnoses: Estimating the clinical utility of assessments of covert awareness in the vegetative state. Brain Inj. 2014, 28, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.R.; Davis, M.H.; Rodd, J.M.; Robson, T.; Ali, A.; Owen, A.M.; Pickard, J.D. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 2009, 132, 2541–2552. [Google Scholar] [CrossRef] [Green Version]


| Authors | Sample | Methods | Findings | Conclusions |
|---|---|---|---|---|
| Autonomic Nervous System | ||||
| Leo et al., 2016 [59] | 12 MCS 10 UWS | GBO of C-LEPs and SR ANS parameters either during a 24-P or following RLS | Only MCS and 2 UWS individuals showed physiological modification of O2 saturation, GBO of C-LEPs and SR either during a 24-P or following RLS | Large-scale ANS parameters and cortical features of advanced pain processing support DOC differential diagnosis and allow identifying residual aware ANS-related cognitive processes |
| Devalle et al., 2018 [69] | 14 UWS 6 MCS | Short-term (<20 s) and long-term (between 20 s and 50 s from noxious stimulus) HRV | Short-term responses in both groups Long-term responses only in MCS | HRV responsiveness differentiates between MCS and UWS |
| Riganello et al., 2019 [70] | 11 MCS 11 UWS 14 HC | HRV assessment using short-term CI | Higher CI in HC compared to DOC at baseline and after noxious stimulation Higher values in MCS versus UWS after noxious stimulation Lower values in noxious versus non-noxious condition in UWS group | UWS have a less complex ANS response to noxious stimuli |
| Luauté et al., 2018 [72] | 7 UWS 6 MCS 7 HC | SCL with stimulations in auditory and olfactory modalities | No different responses in DOC | No DOC distinction |
| Riganello et al., 2015 [16] | 8 UWS | HRV spectrum | Significant correlation between HRV spectral features and CRS-R | The timely variability of ANS tone serves as an indicator for diagnosis and prognosis |
| Venturella et al., 2019 [79] | 21 UWS | ANS responsiveness to touch- and pain-related stimuli | Fronto-parietal activation in both modalities. Increase in delta oscillations, electrodermal activity, and HRV following painful stimuli | Stimuli can capture basic attention orientation and perceptual processes. Only nociceptive stimulation seems entraining cognitive processes at an aware level |
| Laser-Evoked Potentials and Advanced EEG Signal Analyses | ||||
| De Tommaso et al., 2013 [75] | 3 UWS 4 MCS 11HC | LEP SEPs AMN | LEPs in all patients Significant N2 and P2 latency increase No SEPs in all patients but one MCS AMN in all patients | Possible pain processing preservation despite sensory impairment |
| De Tommaso et al., 2015 [76] | 5 UWS 4 MCS 11 HC | LEP multimodal EP | Constant preservation of LEP despite a variable degree of preservation of the other EPs | Possible pain processing preservation despite sensory impairment |
| De Salvo et al., 2015 [78] | 13 UWS 10 MCS | LEP | Lower amplitudes and more delayed in UWS than MCS | LEP features can discriminate between MCS and UWS |
| Naro et al., 2015 [79] | 23 UWS 15 MCS 15 HC | Aδ-LEP C-LEP | Higher amplitudes and less delayed latencies in HC than DOC Higher amplitudes and less delayed latencies in MCS than UWS Some UWS showed only C-LEP | The residual presence of C-LEP should be assessed when Aδ-LEP are missing, because a potential pain experience should be still present in some patients |
| Naro et al., 2015 [80] | 10 UWS 10 HC | MEP LEP PMI | PMI deterioration in DOC, more in UWS than MCS PMI preserved in some UWS | Residual plasticity properties at large-scale cortical level suggesting residual pain awareness |
| Naro et al., 2016 [81] | 18 UWS 15 MCS | GBO following RLS | Increase in GBO power and NCS-R score in HC, MCS and 5 UWS | Presence of aware pain processing as per GBO modulation |
| Aricò et al., 2016 [82] | 8 UWS 6 MCS | LEP 24 h polysomnography | Higher LEP latencies and lower amplitudes in UWS than MCS Spared sleep structure in MCS compared to UWS in correlation with LEP findings | Preserved sleep structure and pain processing require a spared global brain connectivity, which expresses thalamo–cortical functionality supporting consciousness |
| Naro et al., 2017 [83] | 10 UWS 10 MCS 10 HC | IPI variability of LEP components | Correlation between IPI and NCS-R | IPI variability might represent an objective measure of pain processing |
| Calabrò et al., 2017 [84] | 11 UWS 10 MCS | γ-band LORETA activations, GBO, and HRV following RLS | Spared γ-band LORETA activations, GBO, and HRV in MCS and two UWS (with brain activation limited to limbic areas) | Nearly physiologic pain processing in MCS; connectivity breakdown in UWS, which limits aware pain perception to residual |
| Naro et al., 2015 [85] | 10 UWS 10 MCS 10 HC | 1 Hz rTMS over ACC affecting frontal GBO and EEP | Increase in GBO and decrease in EPP in MCS and two UWS subjects Decreased pain rating in HC (as per VAS) and MCS (as per NCS-R) | ACC rTMS aftereffects suggest aware pain processing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, R.S.; Pignolo, L.; Müller-Eising, C.; Naro, A. Pain Perception in Disorder of Consciousness: A Scoping Review on Current Knowledge, Clinical Applications, and Future Perspective. Brain Sci. 2021, 11, 665. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11050665
Calabrò RS, Pignolo L, Müller-Eising C, Naro A. Pain Perception in Disorder of Consciousness: A Scoping Review on Current Knowledge, Clinical Applications, and Future Perspective. Brain Sciences. 2021; 11(5):665. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11050665
Chicago/Turabian StyleCalabrò, Rocco Salvatore, Loris Pignolo, Claudia Müller-Eising, and Antonino Naro. 2021. "Pain Perception in Disorder of Consciousness: A Scoping Review on Current Knowledge, Clinical Applications, and Future Perspective" Brain Sciences 11, no. 5: 665. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11050665