Antidepressant-like Effects of Renin Inhibitor Aliskiren in an Inflammatory Mouse Model of Depression
Abstract
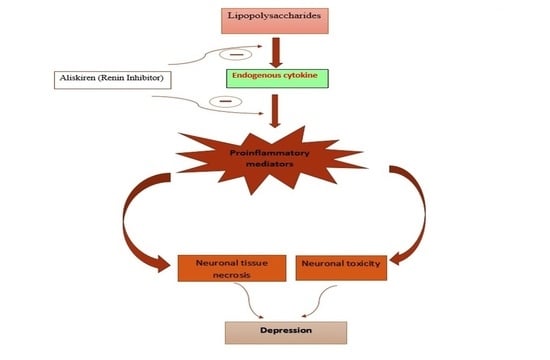
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Chemicals
2.3. Study Design
2.4. Open-Field Test (OFT)
2.5. Tail Suspension Test (TST)
2.6. Forced Swim Test (FST)
2.7. Sucrose Preference Test (SPT)
2.8. Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of Aliskiren on LPS-Induced Sickness Behavior in Mice
Open Field Test
3.2. Effect of Aliskiren on LPS-Induced Depressive-like Behavior in Mice in TST, FST and SPT
3.2.1. Tail Suspension Test
3.2.2. Forced Swim Test
3.2.3. Sucrose Preference Test
3.3. Assessment of Aliskiren Effect on Glial Cell Marker in Prefrontal Cortex
3.4. Assessment of LPS and Aliskiren Effect on Proinflammatory Cytokines in Prefrontal Cortex
3.4.1. Effect of LPS and Aliskiren on TNF-α
3.4.2. Effect of LPS and Aliskiren on Brain IL-1β Levels
3.4.3. Effect of LPS and Aliskiren on Brain IL-6 Levels
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jo, W.K.; Zhang, Y.; Emrich, H.M.; Dietrich, D.E. Glia in the cytokine-mediated onset of depression: Fine tuning the immune response. Front. Cell. Neurosci. 2015, 9, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, T.; Staab, J.; Evans, D.L. Medical co-morbidity in depressive disorders. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2007, 19, 289–303. [Google Scholar] [CrossRef] [PubMed]
- WHO. Media Centre Fact Sheet No. 369. 2012. Available online: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed on 30 March 2022).
- Dwivedi, Y. Emerging role of microRNAs in major depressive disorder: Diagnosis and therapeutic implications. Dialogues Clin. Neurosci. 2014, 16, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in bipolar depression. Front. Psychiatry 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- He, M.C.; Shi, Z.; Sha, N.N.; Chen, N.; Peng, S.Y.; Liao, D.F.; Wong, M.S.; Dong, X.L.; Wang, Y.J.; Yuan, T.F.; et al. Paricalcitol alleviates lipopolysaccharide-induced depressive-like behavior by suppressing hypothalamic microglia activation and neuroinflammation. Biochem. Pharmacol. 2019, 163, 1–8. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Reviews. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef]
- Moieni, M.; Irwin, M.R.; Jevtic, I.; Olmstead, R.; Breen, E.C.; Eisenberger, N.I. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 1709–1716. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol attenuates LPS-induced neuroinflammation, amyloidogenesis, and cognitive impairments via the inhibition of NF-κB in male C57BL/6J mice and BV2 microglial cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Yan, Y.M.; Li, S.Y.; He, D.H.; Xiong, S.; Wei, S.F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.F.; et al. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Patel, R.N.; Thakker, G.D.; Lowenstein, C.J.; Attur, M.G.; Abramson, S.B. Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett. 1997, 410, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Ona, V.O.; Li, M.; Ferrante, R.J.; Fink, K.B.; Zhu, S.; Bian, J.; Guo, L.; Farrell, L.A.; Hersch, S.M. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000, 6, 797–801. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Drozda, M.; Zhang, W.; Stavrovskaya, I.G.; Cattaneo, E.; Ferrante, R.J.; Kristal, B.S.; Friedlander, R.M. Minocycline inhibits caspase-independent and-dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 10483–10487. [Google Scholar] [CrossRef] [Green Version]
- Domercq, M.; Matute, C. Neuroprotection by tetracyclines. Trends Pharmacol. Sci. 2004, 25, 609–612. [Google Scholar] [CrossRef]
- Jordan, J.; Fernandez-Gomez, F.; Ramos, M.; Ikuta, I.; Aguirre, N.; Galindo, M. Minocycline and cytoprotection: Shedding new light on a shadowy controversy. Curr. Drug Deliv. 2007, 4, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Corbacella, E.; Lanzoni, I.; Ding, D.; Previati, M.; Salvi, R. Minocycline attenuates gentamicin induced hair cell loss in neonatal cochlear cultures. Hear. Res. 2004, 197, 11–18. [Google Scholar] [CrossRef]
- Henry, C.J.; Huang, Y.; Wynne, A.; Hanke, M.; Himler, J.; Bailey, M.T.; Sheridan, J.F.; Godbout, J.P. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflamm. 2008, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, B.; Yu, Y.; Zhang, Z.; Guo, F.; Beltz, T.G.; Thunhorst, R.L.; Felder, R.B.; Johnson, A.K. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin-angiotensin system and inflammation. Hypertension 2016, 67, 970–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebre, A.K.; Altaye, B.M.; Atey, T.M.; Tuem, K.B.; Berhe, D.F. Targeting renin-angiotensin system against alzheimer’s disease. Front. Pharmacol. 2018, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Hu, H.; Qiao, Y.; Xu, P.; Yang, M.; Dang, R.; Han, W.; Guo, Y.; Chen, D.; Jiang, P. The involvement of renin-angiotensin system in lipopolysaccharide-induced behavioral changes, neuroinflammation, and disturbed insulin signaling. Front. Pharmacol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Vian, J.; Pereira, C.; Chavarria, V.; Köhler, C.; Stubbs, B.; Quevedo, J.; Kim, S.W.; Carvalho, A.F.; Berk, M.; Fernandes, B.S. The renin-angiotensin system: A possible new target for depression. BMC Med. 2017, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain renin-angiotensin system and microglial polarization: Implications for aging and neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Q.; Zhang, J.-C.; Hashimoto, K. Effects of brilliant blue G on serum tumor necrosis factor-α levels and depression-like behavior in mice after lipopolysaccharide administration. Clin. Psychopharmacol. Neurosci. 2014, 12, 31–36. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Lehnardt, S.; Lachance, C.; Patrizi, S.; Lefebvre, S.; Follett, P.L.; Jensen, F.E.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 2478–2486. [Google Scholar] [CrossRef]
- Lien, E.; Means, T.K.; Heine, H.; Yoshimura, A.; Kusumoto, S.; Fukase, K.; Fenton, M.J.; Oikawa, M.; Qureshi, N.; Monks, B.; et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 2000, 105, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Fu, X.; Han, R.; Liu, X.; Zhao, X.; Wei, J. Neuroprotective effect of HIIT against GFAP hypertrophy through mitochondrial dynamics in APP/PS1 mice. Oxidative Med. Cell. Longev. 2022, 2022, 1764589. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G.; Yasojima, K. Alzheimer disease and neuroinflammation. J. Neural Transmission. Suppl. 2000, 59, 53–57. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [Green Version]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [Green Version]
- Stajic, D.; Selakovic, D.; Jovicic, N.; Joksimovic, J.; Arsenijevic, N.; Lukic, M.L.; Rosic, G. The role of galectin-3 in modulation of anxiety state level in mice. Brain Behav. Immun. 2019, 78, 177–187. [Google Scholar] [CrossRef]
- Benson, S.; Brinkhoff, A.; Lueg, L.; Roderigo, T.; Kribben, A.; Wilde, B.; Witzke, O.; Engler, H.; Schedlowski, M.; Elsenbruch, S. Effects of acute systemic inflammation on the interplay between sad mood and affective cognition. Transl. Psychiatry 2017, 7, 1281. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.D.; Wu, C.L.; Lin, T.K.; Chuang, Y.C.; Yang, D.I. Renin inhibitor aliskiren exerts neuroprotection against amyloid beta-peptide toxicity in rat cortical neurons. Neurochem. Int. 2012, 61, 369–377. [Google Scholar] [CrossRef]
- Attilio, P.J.; Snapper, D.M.; Rusnak, M.; Isaac, A.; Soltis, A.R.; Wilkerson, M.D.; Dalgard, C.L.; Symes, A.J. Transcriptomic analysis of mouse brain after traumatic brain injury reveals that the angiotensin receptor blocker candesartan acts through novel pathways. Front. Neurosci. 2021, 15, 636259. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Lee, S.T.; Kim, S.J.; Song, E.C.; Kim, E.H.; Park, D.K.; Sinn, D.I.; Kim, J.M.; Kim, M.; et al. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J. Pharmacol. Exp. Ther. 2007, 322, 1051–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, N.M.; Kehoe, P.G.; Ben-Shlomo, Y.; Martin, R.M. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J. Alzheimer’s Dis. JAD 2011, 26, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, A.W.; Huang, Y.; O’Connor, J.C.; Dantzer, R.; Kelley, K.W.; Popovich, P.G.; Godbout, J.P. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J. Neuroinflamm. 2010, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Fung, Y.K.; Liu, X.; Pahan, K. Up-regulation of microglial CD11b expression by nitric oxide. J. Biol. Chem. 2006, 281, 14971–14980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wu, M.; Peng, C.; Zhao, G.; Gu, R. GFAP expression in injured astrocytes in rats. Exp. Ther. Med. 2017, 14, 1905–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Yoon, D.; Nam, Y.; Seo, D.; Kim, J.H.; Kim, M.S.; Lee, T.Y.; Kim, K.S.; Ko, P.W.; Lee, H.W.; et al. Lipopolysaccharide administration for a mouse model of cerebellar ataxia with neuroinflammation. Sci. Rep. 2020, 10, 13337. [Google Scholar] [CrossRef]
- McGeer, E.G.; McGeer, P.L. The role of anti-inflammatory agents in Parkinson’s disease. CNS Drugs 2007, 21, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]





| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | GTGGAGTCATACTGGAACATGTA | AATGGTGAAGGTCGGTGT |
| CD11b | TGTCCAGATTGAAGCCATG | CCACAGTTCACACTTCTTTCA |
| GFAP | GCATCTCCACAGTCTTTACC | AACCGCATCACCATTCCT |
| TNF-α | TCTTTGAGATCCATGCCGTT | AGACCCTCACACTCAGATC |
| IL-1β | CTCTTGTTGATGTGCTGCT | GACCTGTTTGAAGTTGAC |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzarea, S.I.; Alhassan, H.H.; Alzarea, A.I.; Al-Oanzi, Z.H.; Afzal, M. Antidepressant-like Effects of Renin Inhibitor Aliskiren in an Inflammatory Mouse Model of Depression. Brain Sci. 2022, 12, 655. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12050655
Alzarea SI, Alhassan HH, Alzarea AI, Al-Oanzi ZH, Afzal M. Antidepressant-like Effects of Renin Inhibitor Aliskiren in an Inflammatory Mouse Model of Depression. Brain Sciences. 2022; 12(5):655. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12050655
Chicago/Turabian StyleAlzarea, Sami I., Hassan H. Alhassan, Abdulaziz I. Alzarea, Ziad H. Al-Oanzi, and Muhammad Afzal. 2022. "Antidepressant-like Effects of Renin Inhibitor Aliskiren in an Inflammatory Mouse Model of Depression" Brain Sciences 12, no. 5: 655. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12050655