Interictal Heart Rate Variability as a Biomarker for Comorbid Depressive Disorders among People with Epilepsy
Abstract
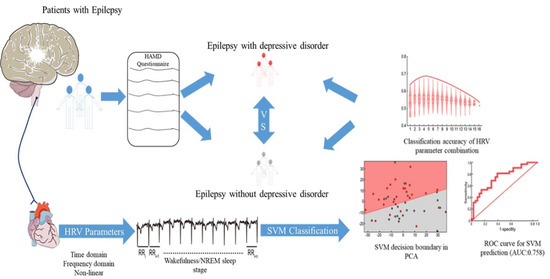
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Diagnosis of Epilepsy and Video-EEG Evaluation
2.2.1. Diagnosis of Epilepsy
2.2.2. Video-EEG Evaluation
2.2.3. Sleep Recording
2.3. Neuropsychological Assessment
2.4. HRV Analysis
2.5. EEG Power Spectral Density Analysis
2.6. Support Vector Machine (SVM) Classification
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Subjects
3.2. Abnormal Heart Rates Are Associated with the Comorbid Depression in PwE
3.3. HRV Parameters, Particularly during NREM Sleep, Are Capable of Classifying PwE with and without Depression
3.4. Combining HRV and EEG PSD Data Further Improves Depression Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Fiest, K.M.; Dykeman, J.; Patten, S.B.; Wiebe, S.; Kaplan, G.G.; Maxwell, C.J.; Bulloch, A.G.; Jette, N. Depression in epilepsy: A systematic review and meta-analysis. Neurology 2013, 80, 590–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanner, A.M.; Byrne, R.; Chicharro, A.; Wuu, J.; Frey, M. A lifetime psychiatric history predicts a worse seizure outcome following temporal lobectomy. Neurology 2009, 72, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, S.; Szoeke, C.E.I.; Jones, N.C.; Salzberg, M.R.; Sheffield, L.J.; Huggins, R.M.; O’Brien, T.J. Neuropsychiatric symptomatology predicts seizure recurrence in newly treated patients. Neurology 2010, 75, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Choi, H.; Hirsch, L.; Katz, A.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017, 76, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Blum, D.; Reed, M.; Fanning, K. The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav. 2003, 4, 515–521. [Google Scholar] [CrossRef]
- Mula, M.; Iudice, A.; La Neve, A.; Mazza, M.; Mazza, S.; Cantello, R.M.; Kanner, A. Validation of the Hamilton Rating Scale for Depression in adults with epilepsy. Epilepsy Behav. 2014, 41, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Mula, M. Developments in depression in epilepsy: Screening, diagnosis, and treatment. Expert Rev. Neurother. 2019, 19, 269–276. [Google Scholar] [CrossRef]
- Heiman, G.A.; Kamberakis, K.; Gill, R.; Kalachikov, S.; Pedley, T.A.; Hauser, W.A.; Ottman, R. Evaluation of depression risk in LGI1 mutation carriers. Epilepsia 2010, 51, 1685–1690. [Google Scholar] [CrossRef] [Green Version]
- Kanner, A.M. Is depression associated with an increased risk of treatment-resistant epilepsy? Research strategies to investigate this question. Epilepsy Behav. 2014, 38, 3–7. [Google Scholar] [CrossRef]
- Lacey, C.J.; Salzberg, M.R.; D’Souza, W.J. Serotonin transporter gene × environment and risk of depression in community-treated epilepsy. Epilepsy Behav. 2014, 39, 33–37. [Google Scholar] [CrossRef]
- Lang, U.E.; Borgwardt, S. Molecular Mechanisms of Depression: Perspectives on New Treatment Strategies. Cell. Physiol. Biochem. 2013, 31, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.H.; Tang, X.C.; Xu, L.H.; Wei, Y.Y.; Hu, Y.G.; Cui, H.R.; Tang, Y.Y.; Chen, T.; Li, C.B.; Zhou, L.L.; et al. Imbalance Model of Heart Rate Variability and Pulse Wave Velocity in Psychotic and Nonpsychotic Disorders. Schizophr. Bull. 2021, 48, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Carnevali, L.; de los Angeles Pico Alfonso, M.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Borrione, L.; Brunoni, A.R.; Sampaio-Junior, B.; Aparicio, L.M.; Kemp, A.; Benseñor, I.; Lotufo, P.A.; Fraguas, R. Associations between symptoms of depression and heart rate variability: An exploratory study. Psychiatry Res. 2018, 262, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [Green Version]
- Dono, F.; Evangelista, G.; Frazzini, V.; Vollono, C.; Carrarini, C.; Russo, M.; Ferrante, C.; Di Stefano, V.; Marchionno, L.P.; De Angelis, M.V.; et al. Interictal Heart Rate Variability Analysis Reveals Lateralization of Cardiac Autonomic Control in Temporal Lobe Epilepsy. Front. Neurol. 2020, 11, 842. [Google Scholar] [CrossRef]
- Lotufo, P.A.; Valiengo, L.; Benseñor, I.M.; Brunoni, A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012, 53, 272–282. [Google Scholar] [CrossRef]
- Romigi, A.; Albanese, M.; Placidi, F.; Izzi, F.; Mercuri, N.B.; Marchi, A.; Liguori, C.; Campagna, N.; Duggento, A.; Canichella, A.; et al. Heart rate variability in untreated newly diagnosed temporal lobe epilepsy: Evidence for ictal sympathetic dysregulation. Epilepsia 2016, 57, 418–426. [Google Scholar] [CrossRef]
- Arbune, A.A.; Jeppesen, J.; Conradsen, I.; Ryvlin, P.; Beniczky, S. Periictal heart rate variability parameters as surrogate markers of seizure severity. Epilepsia 2020, 61, S55–S60. [Google Scholar] [CrossRef]
- Myers, A.K.; Sivathamboo, S.; Perucca, P. Heart rate variability measurement in epilepsy: How can we move from research to clinical practice? Epilepsia 2018, 59, 2169–2178. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.X.; Milaneschi, Y.; Lamers, F.; Nolte, I.M.; Snieder, H.; Dolan, C.V.; Penninx, B.W.J.H.; de Geus, E. The association of depression and anxiety with cardiac autonomic activity: The role of confounding effects of antidepressants. Depress. Anxiety 2019, 36, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Buchsbaum, R.; Resor, S.; Hirsch, L. Psychiatric and behavioral side effects of the newer antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2007, 10, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Sugita, Y.; Koga, E.; Shirakawa, S.; Inoue, K.; Uchida, S.; Kuwahara, H.; Kousaka, M.; Kobayashi, T.; Tsuji, Y.; et al. Proposed supplements and amendments to ‘A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin. Neurosci. 2001, 55, 305–310. [Google Scholar]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton depression rating scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Dong, F.; Du, Y.; Shen, J.; Ding, S.; Wang, L.; Ye, M.; Wang, Y.; Xia, N.; et al. Validation of the Chinese version of the Hamilton Rating Scale for Depression in adults with epilepsy. Epilepsy Behav. 2018, 89, 148–152. [Google Scholar] [CrossRef]
- Katzman, R.; Zhang, M.; Wang, Z.; Liu, W.T.; Yu, E.; Wong, S.-C.; Salmon, D.P.; Grant, I. A Chinese version of the mini-mental state examination; Impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988, 41, 971–978. [Google Scholar] [CrossRef]
- Herzig, D.; Eser, P.; Omlin, X.; Riener, R.; Wilhelm, M.; Achermann, P. Reproducibility of Heart Rate Variability Is Parameter and Sleep Stage Dependent. Front. Physiol. 2018, 8, 01100. [Google Scholar] [CrossRef] [Green Version]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Georgieva-Tsaneva, G.; Gospodinova, E.; Gospodinov, M.; Cheshmedzhiev, K. Cardio-Diagnostic Assisting Computer System. Diagnostics 2020, 10, 322. [Google Scholar] [CrossRef]
- Karemaker, J.M. Autonomic integration: The physiological basis of cardiovascular variability. J. Physiol. 1999, 517 Pt 2, 316. [Google Scholar] [CrossRef] [PubMed]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. Handb. Clin. Neurol. 2013, 117, 379–393. [Google Scholar] [PubMed]
- Toichi, M.; Sugiura, T.; Murai, T.; Sengoku, A. A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R–R interval. J. Auton. Nerv. Syst. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Cherkassky, V. The Nature of Statistical Learning Theory. IEEE Trans. Neural Netw. 1997, 8, 1564. [Google Scholar] [CrossRef] [Green Version]
- Morita, S. Chemometrics and Related Fields in Python. Anal. Sci. 2020, 36, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Taralov, Z.Z.; Terziyski, K.; Kostianev, S.S. Heart Rate Variability as a Method for Assessment of the Autonomic Nervous System and the Adaptations to Different Physiological and Pathological Conditions. Folia Medica 2016, 57, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Adelöw, C.; Andersson, T.; Ahlbom, A.; Tomson, T. Hospitalization for psychiatric disorders before and after onset of unprovoked seizures/epilepsy. Neurology 2012, 78, 396–401. [Google Scholar] [CrossRef]
- Hesdorffer, D.C.; Ishihara, L.; Mynepalli, L.; Webb, D.J.; Weil, J.; Hauser, W.A. Epilepsy, suicidality, and psychiatric disorders: A bidirectional association. Ann. Neurol. 2012, 72, 184–191. [Google Scholar] [CrossRef]
- Kanner, A.M. Depression and epilepsy: A bidirectional relation? Epilepsia 2011, 52 (Suppl. 1), 21–27. [Google Scholar] [CrossRef]
- Benarroch, E.E. The Central Autonomic Network: Functional Organization, Dysfunction, and Perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]




| n | Gender (F/M) | p | Age (Year, Mean ± SD) | p | |
|---|---|---|---|---|---|
| PwE with depression | 60 | 32/28 | 0.630 | 50.17 ± 15.23 | 0.099 |
| PwE without depression | 51 | 23/28 | 43.69 ± 17.6 | ||
| Non-epilepsy controls | 47 | 25/22 | 49.79 ± 16 |
| Variables | Without Depression (n = 51) | With Depression (n = 60) | p-Value | |
|---|---|---|---|---|
| Seizure characteristics | Seizure Origin | 0.486 | ||
| Temporal lobe | 27 | 37 | ||
| Frontal lobe | 5 | 6 | ||
| Occipital lobe | 1 | 3 | ||
| Unknown origin | 18 | 14 | ||
| Mean duration of disease (Year, mean ± SD) | 7.65 ± 9.7 | 10.03 ± 14.5 | 0.652 | |
| Seizure frequency | 0.035 | |||
| per week | 1 | 7 | ||
| per month | 16 | 8 | ||
| per year | 23 | 34 | ||
| no seizure in a year | 11 | 11 | ||
| AEDs information | Taking AEDs | 46/51 | 53/60 | 0.753 |
| Number of AEDs (mean ± SD) | 1.24 ± 0.86 | 1.23 ± 0.83 | 0.677 | |
| LEV | 14/51 | 18/60 | 0.835 | |
| MMSE score (mean ± SD) | 28.3 ± 1.9 | 27.2 ± 2.3 | 0.004 | |
| Stage | HRV Parameters | Non-Epilepsy Controls (n = 47) | PwE without Depression (n = 51) | PwE with Depression (n = 60) | p-Value | Post-Hoc (Bonferroni Corrections) |
|---|---|---|---|---|---|---|
| Wakefulness stage | HR | 66.4 ± 9.7 | 67.5 ± 9.6 | 71.3 ± 10.3 | 0.0421 | all p’s > 0.05 |
| SDNN (msec.) | 46.9 ± 19.6 | 44.4 ± 17.3 | 34.4 ± 14.3 | <0.0001 | p2, p3 < 0.001, p1 = 1 | |
| RMSSD (msec.) | 32.0 ± 14.8 | 32.0 ± 14.5 | 24.8 ± 12.9 | 0.0006 | p2, p3 < 0.05, p1 = 1 | |
| PNN50 | 10.7 ± 11.2 | 12.7 ± 12.6 | 6.6 ± 11.0 | 0.0001 | p2, p3 < 0.05, p1 = 1 | |
| LF | 55.9 ± 18.9 | 51.8 ± 16.6 | 53.7 ± 18.7 | 0.4602 | all p > 0.05 | |
| HF | 48.0 ± 17.2 | 48.1 ± 16.6 | 46.3 ± 18.8 | 0.6197 | all p > 0.05 | |
| LF/HF | 1.8 ± 1.6 | 1.7 ± 1.7 | 1.9 ± 1.9 | 0.6242 | all p > 0.05 | |
| ApEn | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.8226 | all p > 0.05 | |
| SD1 | 22.8 ± 9.9 | 23.3 ± 10.8 | 17.9 ± 9.9 | 0.004 | p2, p3 < 0.05, p1 = 1 | |
| SD2 | 57.1 ± 19.9 | 56.4 ± 23.6 | 43.5 ± 18.8 | 0.003 | p2, p3 < 0.05, p1 = 1 | |
| SD1/SD2 | 0.42 ± 0.15 | 0.44 ± 0.19 | 0.43 ± 0.21 | 0.823 | all p’s > 0.05 | |
| Non-rapid eye movement (NREM) sleep stage | HR | 62.8 ± 9.0 | 62.5 ± 9.6 | 67.9 ± 9.5 | 0.0186 | p2 < 0.05, p1, p3 > 0.05 |
| SDNN (msec.) | 47.9 ± 17.7 | 48.4 ± 18.0 | 38.9 ± 16.3 | 0.0153 | p2, p3 < 0.05, p1 = 1 | |
| RMSSD (msec.) | 40.8 ± 20.9 | 41.3 ± 20.4 | 31.6 ± 15.9 | 0.0180 | p2, p3 < 0.05, p1 = 1 | |
| PNN50 | 18.7 ± 17.1 | 21.6 ± 18.8 | 11.8 ± 14.1 | 0.0107 | p2, p3 < 0.05, p1 = 1 | |
| LF | 47.1 ± 17.6 | 45.1 ± 19.2 | 47.9 ± 17.9 | 0.6908 | all p > 0.05 | |
| HF | 52.9 ± 17.6 | 54.9 ± 19.2 | 52.0 ± 17.9 | 0.6864 | all p > 0.05 | |
| LF/HF | 1.2 ± 0.9 | 1.2 ± 1.2 | 1.2 ± 1.0 | 0.6901 | all p > 0.05 | |
| ApEn | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.4881 | all p > 0.05 | |
| SD1 | 28.34 ± 15.1 | 29.4 ± 14.6 | 23.4 ± 12.6 | 0.0396 | p2 < 0.05, p1, p3 > 0.05 | |
| SD2 | 55.5 ± 22.9 | 61.3 ± 24.9 | 50.6 ± 23.4 | 0.1121 | all p > 0.05 | |
| SD1/SD2 | 0.53 ± 0.19 | 0.51 ± 0.19 | 0.47 ± 0.17 | 0.4673 | all p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimaier, G.; Qian, K.; Zheng, Z.; Peng, W.; Zhang, Z.; Ding, J.; Wang, X. Interictal Heart Rate Variability as a Biomarker for Comorbid Depressive Disorders among People with Epilepsy. Brain Sci. 2022, 12, 671. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12050671
Aimaier G, Qian K, Zheng Z, Peng W, Zhang Z, Ding J, Wang X. Interictal Heart Rate Variability as a Biomarker for Comorbid Depressive Disorders among People with Epilepsy. Brain Sciences. 2022; 12(5):671. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12050671
Chicago/Turabian StyleAimaier, Guliqiemu, Kun Qian, Zishuo Zheng, Weifeng Peng, Zhe Zhang, Jing Ding, and Xin Wang. 2022. "Interictal Heart Rate Variability as a Biomarker for Comorbid Depressive Disorders among People with Epilepsy" Brain Sciences 12, no. 5: 671. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12050671