Post-Craniopharyngioma and Cranial Nerve-VI Palsy Update on a MS Patient with Major Depression and Concurrent Neuroimmune Conditions
Abstract
:1. Introduction
- Quelling pain and disability.
- Enduring medication side effects and noting what worked and what stopped working after a while.
- Subduing MD with bupropion, and supplements S-adenosylmethionine (SAMe) and vitamin-D3 (vit-D3).
- Maintaining routines for all medications, self-hypnosis, yoga, and physical exercises to stay fit and lucid.
- And academically studying symptoms and potential remedies for his ailments to engage in physician-assisted autoexperiments to discover solutions that provide him lasting relief [1].
- During his craniopharyngioma diagnosis and bitemporal vision loss.
- Through fractionated stereotactic radiation treatments (FSRT) to shrink his perisellar tumor and regain peripheral vision.
- And in his bout with cranial nerve-VI (CN6) palsy, diplopia, and their resolution.
2. Case Report
- Academically study his immune-related illnesses through university courses, textbooks, and journals.
- Maintain regular depression inventories.
- Have consistent routines for yoga and self-hypnosis, sleep, and fitness exercises.
- Quell pain and associated anger with yoga and self-hypnosis, and use NSAIDs when pain becomes disabling.
- Judiciously use prescribed medications and note what worked and what aggravated liver enzyme levels, skin rashes, or other side reactions.
- And study new literature methods that could improve health, mood, and physical and cognitive functioning to engage in physician-assisted autoexperiments that afford sustained relief.
3. Materials and Methods
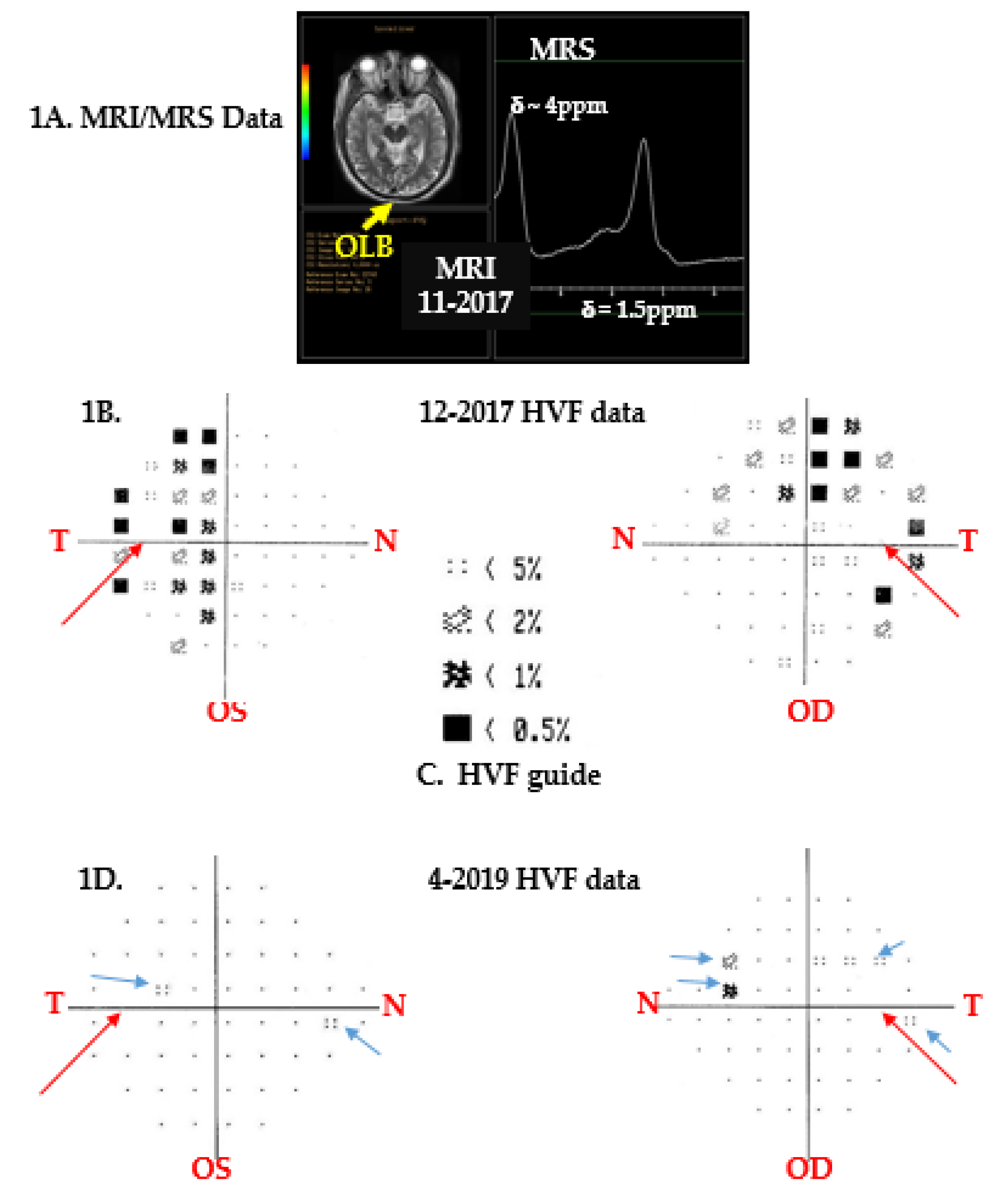
3.1. Brain Imaging and Spectroscopy
3.2. Vision Changes
4. Results and Discussion
4.1. Brain Imaging and Spectroscopy
4.2. Vision Changes
4.3. Bout with CN6 Palsy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Dedication
Conflicts of Interest
References
- Sachinvala, N.D.; Stergiou, A.; Haines, D.E. Remitting long-standing major depression in a multiple sclerosis patient with several concurrent conditions. Neuropsychiatr. Dis. Treat. 2018, 14, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, Z.; Kralickova, P.; Pejchalova, A.; Bloomfield, M.; Nechvatalova, J.; Vlkova, M.; Litzman, J. Selective IgM Deficiency: Clinical and Laboratory Features of 17 Patients and a Review of the Literature. J. Clin. Immunol. 2017, 37, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Kose-Ozlece, H.; Ilik, F.; Huseyinoglu, N. Coexistence of Ehlers-Danlos syndrome and multiple sclerosis. Iran. J. Neurol. 2015, 14, 116–117. [Google Scholar] [PubMed]
- Tripathy, V.; Reddy, B.M. Present status of understanding on the G6PD deficiency and natural selection. J. Postgrad. Med. 2007, 53, 193–202. [Google Scholar]
- Naser Moghadasi, A. Multiple sclerosis in Parsis: A historical issue. Iran. J. Public Health 2014, 43, 387–388. [Google Scholar]
- López, S.; Thomas, M.G.; van Dorp, L.; Ansari-Pour, N.; Stewart, S.; Jones, A.L.; Jelinek, E.; Chikhi, L.; Parfitt, T.; Bradman, N.; et al. The Genetic Legacy of Zoroastrianism in Iran and India: Insights into Population Structure, Gene Flow, and Selection. Am. J. Hum. Genet. 2017, 101, 353–368. [Google Scholar] [CrossRef] [Green Version]
- Ahmadian-Attari, M.M.; Ahmadiani, A.; Kamalinejad, M.; Dargahi, L.; Shirzad, M.; Mosaddegh, M. Treatment of Alzheimer’s disease in Iranian traditional medicine. Iran. Red Crescent Med. J. 2014, 17, e18052. [Google Scholar] [CrossRef]
- Vazquez-Vidal, I.; Chittoor, G.; Laston, S.; Puppala, S.; Kayani, Z.; Mody, K.; Comuzzie, A.G.; Cole, S.A.; Voruganti, V.S. Assessment of cardiovascular disease risk factors in a genetically homogenous population of Parsi Zoroastrians in the United States: A pilot study. Am. J. Hum. Biol. 2016, 28, 440–443. [Google Scholar] [CrossRef]
- Available online: https://patentscope.wipo.int/search/en/ and then type Sachinvala (accessed on 12 October 2019).
- Benedict, R.H.; Fishman, I.; McClellan, M.M.; Bakshi, R.; Weinstock-Guttman, B. Validity of the Beck Depression Inventory-Fast Screen in multiple sclerosis. Mult. Scler. J. 2003, 9, 393–396. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Langhorst, J.; Dobos, G. Yoga for depression: A systematic review and meta-analysis. Depress. Anxiety 2013, 30, 1068–1083. [Google Scholar] [CrossRef]
- Otani, A. Eastern meditative techniques and hypnosis: A new synthesis. Am. J. Clin. Hypn. 2003, 46, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Tiers, M. Integrative Hypnosis a Comprehensive Course in Change; Melissa Tiers Publisher: New York, NY, USA, 2010. [Google Scholar]
- Stetka, B. Available online: https://www.scientificamerican.com/article/do-vitamins-and-supplements-make-antidepressants-more-effective/ (accessed on 26 April 2016).
- Osborn, A.G.; Salzman, K.L.; Jhaveri, M.D. Craniopharyngioma. In Diagnostic Imaging: Brain, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 1048–1051. [Google Scholar]
- Einstien, A.; Virani, R.A. Clinical Relevance of Single-Voxel (1) H MRS Metabolites in Discriminating Suprasellar Tumors. J. Clin. Diagn. Res. 2016, 10, TC01–TC04. [Google Scholar] [CrossRef] [PubMed]
- Chernov, M.F.; Kawamata, T.; Amano, K.; Ono, Y.; Suzuki, T.; Nakamura, R.; Muragaki, Y.; Iseki, H.; Kubo, O.; Hori, T.; et al. Possible role of single-voxel (1) H-MRS in differential diagnosis of suprasellar tumors. J. Neuro-Oncology 2009, 91, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Saeger, W. New aspects of tumor pathology of the pituitary. Pathologe 2015, 36, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.J.; McCallum, E.; Hawkins, R.O.; Stephenson, E.; Vicencio, K. The effects of two assistive technologies on reading comprehension accuracy and rate. Assist. Technol. 2019, 31, 220–230. [Google Scholar] [CrossRef] [PubMed]
- de Voogd, E.L.; de Hullu, E.; Burnett-Heyes, S.; Blackwell, S.E.; Wiers, R.W.; Salemink, E. Imagine the bright side of life: A randomized controlled trial of two types of interpretation bias modification procedure targeting adolescent anxiety and depression. PLoS ONE 2017, 12, e0181147. [Google Scholar] [CrossRef]
- Barber, S.M.; Teh, B.S.; Baskin, D.S. Fractionated Stereotactic Radiotherapy for Pituitary Adenomas: Single-Center Experience in 75 Consecutive Patients. Neurosurgery 2016, 79, 406–417. [Google Scholar] [CrossRef]
- Koch, K.; Schultz, C.C. Clinical and pathogenetic implications of occipital bending in depression. Brain 2014, 137, 1576–1578. [Google Scholar] [CrossRef] [Green Version]
- Takis, A.; Alonistiotis, D.; Ioannou, N.; Mitsopoulou, M.; Papaconstantinou, D. Follow-up of the retinal nerve fiber layer thickness of diabetic patients type 2, as a predisposing factor for glaucoma compared to normal subjects. Clin. Ophthalmol. 2017, 11, 1135–1141. [Google Scholar] [CrossRef]
- Henricsson, M.; Heijl, A. Visual fields at different stages of diabetic retinopathy. Acta Ophthalmol. 1994, 72, 560–569. [Google Scholar] [CrossRef]
- Haines, D.E. Neuroanatomy in Clinical Context: An Atlas of Structures, Sections, Systems, and Syndromes; 9ED; Walter Kluwer Health: Philadelphia, PA, USA, 2015. [Google Scholar]
- Azarmina, M.; Azarmina, H. The six syndromes of the sixth cranial nerve. J. Ophthalmic Vis. Res. 2013, 8, 160–171. [Google Scholar] [PubMed]
- Wijnen, M.; Olsson, D.S.; van den Heuvel-Eibrink, M.M.; Hammarstrand, C.; Janssen, J.A.M.J.L.; van der Lely, A.J.; Johannsson, G.; Neggers, S.J.C.M.M. Excess morbidity and mortality in patients with craniopharyngioma: A hospital-based retrospective cohort study. Eur. J. Endocrinol. 2018, 178, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Weblink Cover Tests. Available online: https://www.aao.org/bcscsnippetdetail.aspx?id=5051fd44-4106-4b1a-bf19-00566baa0b07 (accessed on 12 October 2019).
- Siu, J.; Sharma, S.; Sowerby, L. Unilateral isolated sphenoid sinusitis with contralateral abducens nerve palsy—A rare complication treated in a low-resource setting. J. Otolaryngol. Head Neck Surg. 2015, 1, 44–49. [Google Scholar] [CrossRef] [PubMed]

| Right Eye (OD) Dec-2017 | |||||||
|---|---|---|---|---|---|---|---|
| Sector | T | TS | TI | G | N | NS | NI |
| Patient | 85 | 176 | 136 | 101 | 72 | 93 | 91 |
| Statistic | 72 | 131 | 138 | 96 | 72 | 102 | 104 |
| Overall Patient OD RNFLT: S = 135; I = 114; T 85; and N = 72 | |||||||
| Left Eye (OS) Dec-2017 | |||||||
| Patient | 44 | 124 | 126 | 90 | 82 | 116 | 99 |
| Statistic | 72 | 131 | 138 | 96 | 72 | 102 | 104 |
| Overall Patient OS RNFLT: S = 120; I = 112; T = 44; N = 82 | |||||||
| Right Eye (OD) Apr-2019 | |||||||
| Sector | T | TS | TI | G | N | NS | NI |
| Patient | 55 | 127 | 128 | 87 | 70 | 94 | 94 |
| Statistic | 72 | 131 | 138 | 96 | 72 | 102 | 104 |
| Overall Patient OD RNFLT: S = 111; I = 111; T = 55; and N = 70 | |||||||
| Left Eye (OS) Apr-2019 | |||||||
| Patient | 40 | 117 | 119 | 80 | 63 | 102 | 98 |
| Statistic | 72 | 131 | 138 | 96 | 72 | 102 | 104 |
| Overall Patient OS RNFLT: S = 109; I = 108; T = 40; and N = 63 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachinvala, N.D.; Stergiou, A.; Haines, D.E.; Kocharian, A.; Lawton, A. Post-Craniopharyngioma and Cranial Nerve-VI Palsy Update on a MS Patient with Major Depression and Concurrent Neuroimmune Conditions. Brain Sci. 2019, 9, 281. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci9100281
Sachinvala ND, Stergiou A, Haines DE, Kocharian A, Lawton A. Post-Craniopharyngioma and Cranial Nerve-VI Palsy Update on a MS Patient with Major Depression and Concurrent Neuroimmune Conditions. Brain Sciences. 2019; 9(10):281. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci9100281
Chicago/Turabian StyleSachinvala, Navzer D., Angeline Stergiou, Duane E. Haines, Armen Kocharian, and Andrew Lawton. 2019. "Post-Craniopharyngioma and Cranial Nerve-VI Palsy Update on a MS Patient with Major Depression and Concurrent Neuroimmune Conditions" Brain Sciences 9, no. 10: 281. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci9100281




