Frontal Alpha Asymmetry and Inhibitory Control among Individuals with Cannabis Use Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
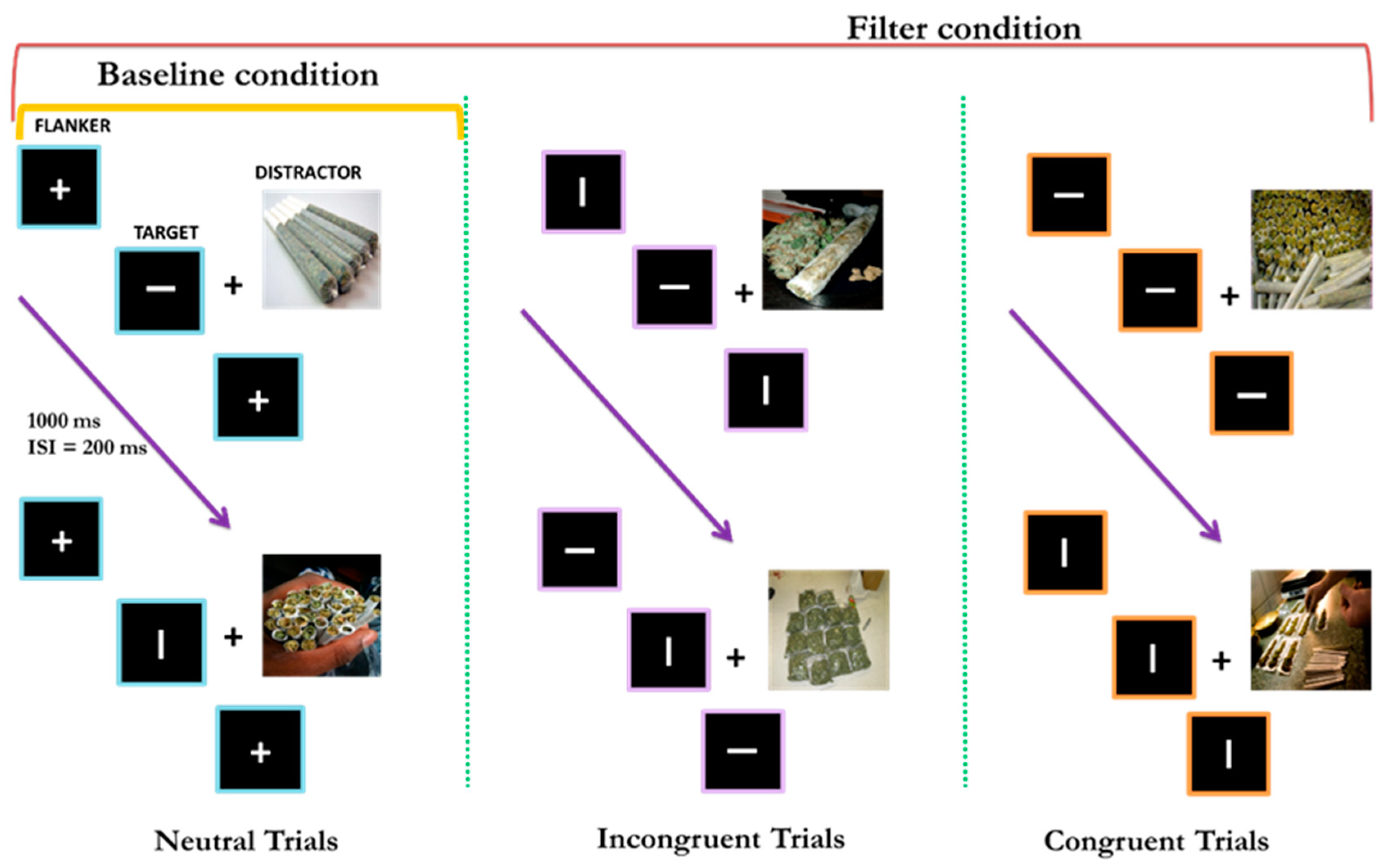
2.2. Materials and Procedure
2.3. Data Recording and Analysis
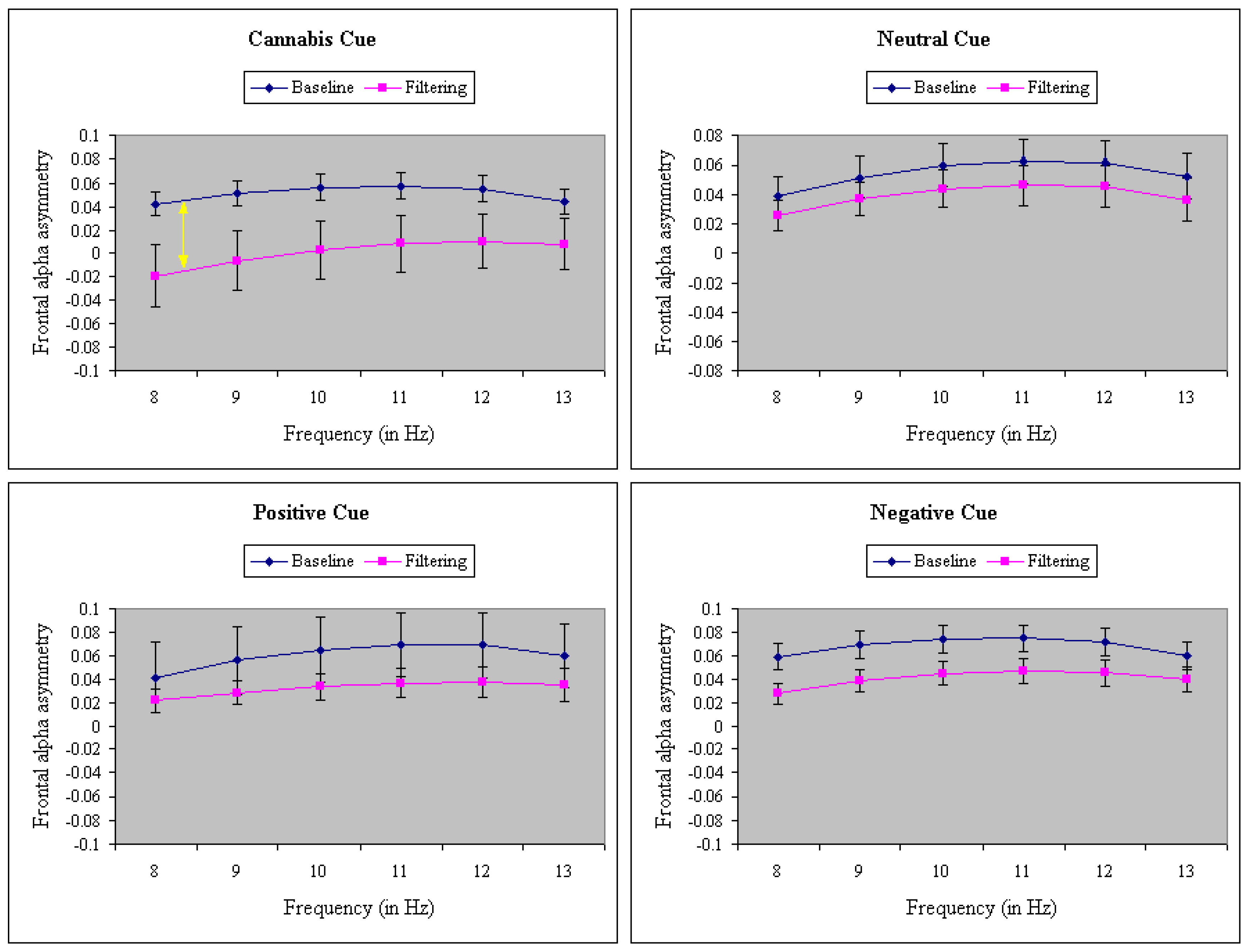
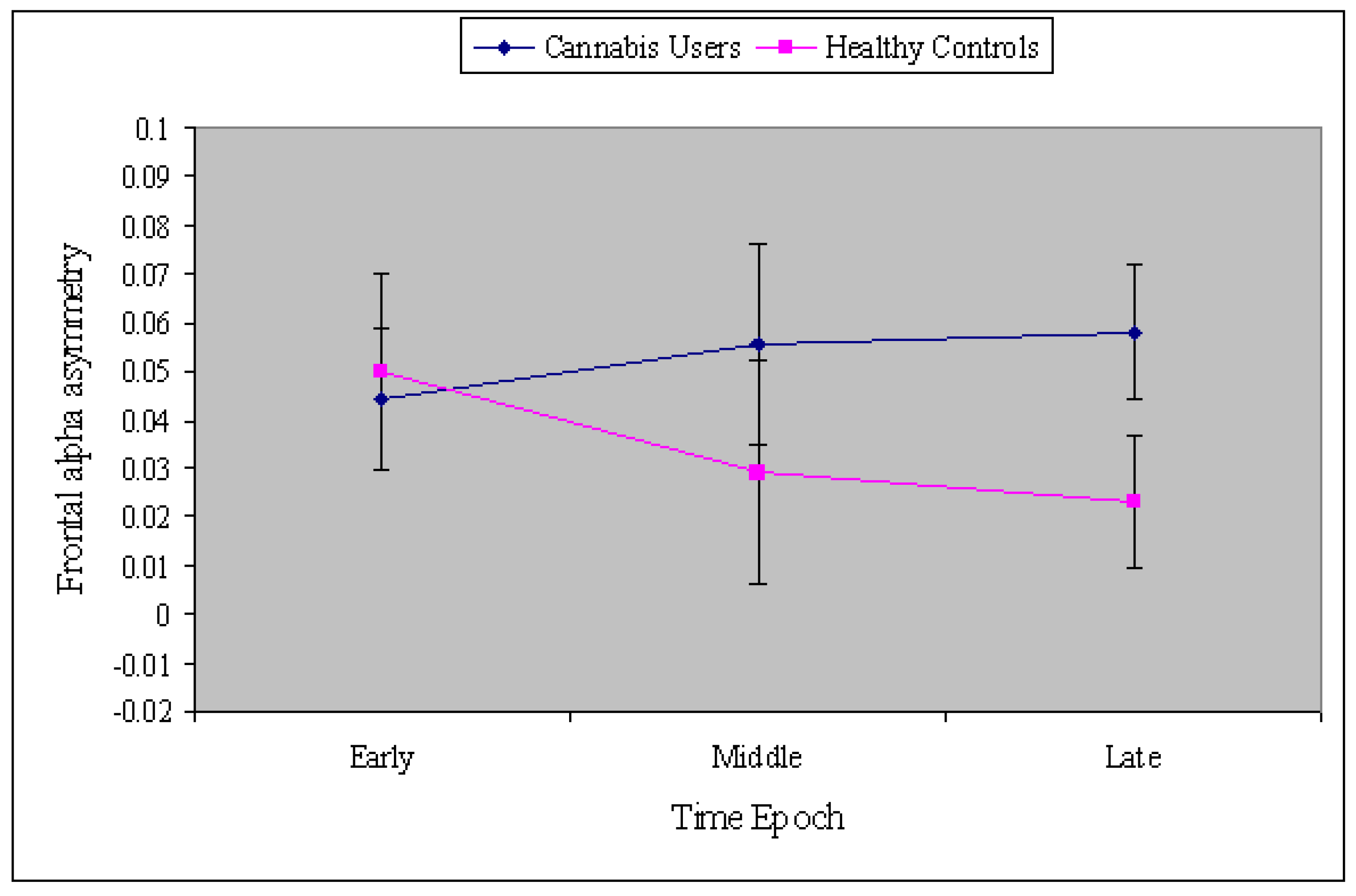
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azofeifa, A.; Schauer, G.; McAfee, T.; Grant, A.; Lyerla, R.; Mattson, M.E. National Estimates of Marijuana Use and Related Indicators—National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill. Summ. 2016, 65, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Carliner, H.; Brown, Q.L.; Sarvet, A.L.; Hasin, D.S. Cannabis use, attitudes, and legal status in the U.S.: A review. Prev. Med. 2017, 104, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Okaneku, J.; Vearrier, D.; McKeever, R.G.; LaSala, G.S.; Greenberg, M.I. Change in perceived risk associated with marijuana use in the United States from 2002 to 2012. Clin. Toxicol. 2015, 53, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Susan, R.B. Adverse Health Effects of Marijuana Use. N. Engl. J. Med. 2016, 370, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Swanson, J.M.; Evins, A.E.; DeLisi, L.E.; Meier, M.H.; González, R.; Bloomfield, M.A.P.; Curran, H.V.; Baler, R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 2016, 73, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Degenhardt, L. The adverse health effects of chronic cannabis use. Drug Test. Anal. 2014, 6, 39–45. [Google Scholar] [CrossRef]
- Arterberry, B.J.; Padovano, H.T.; Foster, K.T.; Zucker, R.A.; Hicks, B.M. Higher average potency across the United States is associated with progression to first cannabis use disorder symptom. Drug Alcohol. Depend. 2019, 195, 186–192. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef]
- Hasin, D.S.; Saha, T.D.; Kerridge, B.T.; Goldstein, R.B.; Chou, S.P.; Zhang, H.; Jung, J.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; et al. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry 2015, 72, 1235–1242. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Field, M.; Cox, W. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol. Depend. 2008, 97, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S.B.T.; Coventry, K. A Dual-Process Approach to Behavioral Addiction: The Case of Gambling. In Handbook of Implicit Cognition and Addiction; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2006; pp. 29–44. [Google Scholar]
- Watson, P.; De Wit, S.; Hommel, B.; Wiers, R.W. Motivational Mechanisms and Outcome Expectancies Underlying the Approach Bias toward Addictive Substances. Front. Psychol. 2012, 3, 440. [Google Scholar] [CrossRef] [Green Version]
- Wills, T.A.; Bantum, E.O.; Pokhrel, P.; Maddock, J.E.; Ainette, M.G.; Morehouse, E.; Fenster, B. A dual-process model of early substance use: Tests in two diverse populations of adolescents. Health Psychol. 2013, 32, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The incentive sensitization theory of addiction: Some current issues. Philos. Trans. R. Soc. B Boil. Sci. 2008, 363, 3137–3146. [Google Scholar] [CrossRef]
- Wiers, R.W.; Bartholow, B.D.; Wildenberg, E.V.D.; Thush, C.; Engels, R.C.; Sher, K.J.; Grenard, J.; Ames, S.L.; Stacy, A.W. Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacol. Biochem. Behav. 2007, 86, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 2018, 55, e12879. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Boil. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef]
- Davidson, R.J.; Ekman, P.; Saron, C.D.; Senulis, J.A.; Friesen, W.V. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. J. Pers. Soc. Psychol. 1990, 58, 330–341. [Google Scholar] [CrossRef]
- Blackhart, G.C.; Minnix, J.A.; Kline, J.P. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biol. Psychol. 2006, 72, 46–50. [Google Scholar] [CrossRef]
- Papousek, I.; Reiser, E.M.; Weber, B.; Freudenthaler, H.H.; Schulter, G. Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology 2012, 49, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.K.; Davidson, R.J. Prefrontal Brain Asymmetry: A Biological Substrate of the Behavioral Approach and Inhibition Systems. Psychol. Sci. 1997, 8, 204–210. [Google Scholar] [CrossRef]
- Wheeler, R.E.; Davidson, R.J.; Tomarken, A.J. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology 2007, 30, 82–89. [Google Scholar] [CrossRef]
- Smith, E.E.; Reznik, S.J.; Stewart, J.L.; Allen, J.J.B. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017, 111, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Coan, J.A.; Allen, J.J.; Harmon-Jones, E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology 2001, 38, 912–925. [Google Scholar] [CrossRef]
- Killeen, L.A.; Teti, D.M. Mothers’ frontal EEG asymmetry in response to infant emotion states and mother–infant emotional availability, emotional experience, and internalizing symptoms. Dev. Psychopathol. 2012, 24, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Cerebral asymmetry and emotion: Conceptual and methodological conundrums. Cogn. Emot. 1993, 7, 115–138. [Google Scholar] [CrossRef]
- Allen, J.J.; Reznik, S.J. Frontal EEG Asymmetry as a Promising Marker of Depression Vulnerability: Summary and Methodological Considerations. Curr. Opin. Psychol. 2015, 4, 93–97. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology 1998, 35, 607–614. [Google Scholar] [CrossRef]
- Mathersul, D.; Williams, L.M.; Hopkinson, P.J.; Kemp, A.H. Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion 2008, 8, 560–572. [Google Scholar] [CrossRef]
- Ellis, A.J.; Kinzel, C.; Salgari, G.C.; Loo, S.K. Frontal Alpha Asymmetry Predicts Inhibitory Processing in Youth with Attention Deficit/Hyperactivity Disorder. Neuropsychologia 2017, 102, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Keune, P.M.; Wiedemann, E.; Schneidt, A.; Schönenberg, M. Frontal brain asymmetry in adult attention-deficit/hyperactivity disorder (ADHD): Extending the motivational dysfunction hypothesis. Clin. Neurophysiol. 2015, 126, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Gable, P.A.; Mechin, N.C.; Neal, L.B. Booze cues and attentional narrowing: Neural correlates of virtual alcohol myopia. Psychol. Addict. Behav. 2016, 30, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bowley, C.; Faricy, C.; Hegarty, B.; Johnstone, S.J.; Smith, J.L.; Kelly, P.J.; Rushby, J.A. The effects of inhibitory control training on alcohol consumption, implicit alcohol-related cognitions and brain electrical activity. Int. J. Psychophysiol. 2013, 89, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Knott, V.J.; Naccache, L.; Cyr, E.; Fisher, D.J.; McIntosh, J.F.; Millar, A.M.; Villeneuve, C.M. Craving-Induced EEG Reactivity in Smokers: Effects of Mood Induction, Nicotine Dependence and Gender. Neuropsychobiology 2008, 58, 187–199. [Google Scholar] [CrossRef]
- Ruglass, L.M.; Shevorykin, A.; Dambreville, N.; Melara, R.D. Neural and behavioral correlates of attentional bias to cannabis cues among adults with cannabis use disorders. Psychol. Addict. Behav. 2019, 33, 69–80. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Philadelphia, PA, USA, 2000. [Google Scholar]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Melara, R.D.; Singh, S.; Hien, D.A. Neural and Behavioral Correlates of Attentional Inhibition Training and Perceptual Discrimination Training in a Visual Flanker Task. Front. Hum. Neurosci. 2018, 12, 191. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings; The Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA, 1999. [Google Scholar]
- Garner, W.R. The stimulus in information processing. In Sensation and Measurement: Papers in Honor of S.S. Stevens; Moskowitz, H.R., Scharf, B., Stevens, J.C., Eds.; Springer: New York, NY, USA, 1974; pp. 77–90. [Google Scholar]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Gasser, T.; Bächer, P.; Möcks, J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr. Clin. Neurophysiol. 1982, 53, 119–124. [Google Scholar] [CrossRef]
- Quaedflieg, C.W.; Smulders, F.T.; Meyer, T.; Peeters, F.; Merckelbach, H.; Smeets, T. The validity of individual frontal alpha asymmetry EEG neurofeedback. Soc. Cogn. Affect. Neurosci. 2016, 11, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Quaedflieg, C.W.E.M.; Giesbrecht, T.; Meijer, E.H.; Abiad, S.; Smeets, T. Frontal EEG asymmetry as predictor of physiological responses to aversive memories. Psychophysiology 2014, 51, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Chapman, J.P.; Chapman, L.J.; Henriques, J.B. Asymmetrical Brain Electrical Activity Discriminates Between Psychometrically-Matched Verbal and Spatial Cognitive Tasks. Psychophysiology 1990, 27, 528–543. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Pattij, T.; Wiskerke, J.; Schoffelmeer, A.N. Cannabinoid modulation of executive functions. Eur. J. Pharmacol. 2008, 585, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Cousijn, J.; Goudriaan, A.E.; Ridderinkhof, K.R.; van den Brink, W.; Veltman, D.J.; Wiers, R.W. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2013, 18, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Mechin, N.; Gable, P.A.; Hicks, J.A. Frontal asymmetry and alcohol cue reactivity: Influence of core personality systems. Psychophysiology 2016, 53, 1224–1231. [Google Scholar] [CrossRef]
- Sjoerds, Z.; Brink, W.V.D.; Beekman, A.T.F.; Penninx, B.W.J.H.; Veltman, D.J. Cue Reactivity Is Associated with Duration and Severity of Alcohol Dependence: An fMRI Study. PLoS ONE 2014, 9, e84560. [Google Scholar] [CrossRef]

| Variable | CUD (n = 20) M (SD) or % | Controls (n = 20) M (SD) or % | t statistics/χ2 |
|---|---|---|---|
| Baseline Session | |||
| Age | 26.2 (8.53) | 28 (10.87) | t = 0.58, p = 0.56 |
| Education1 | 13.85 (1.63) | 14.85 (1.35) | t = 2.11, p = 0.041 |
| Sex (% males) | 80% | 75% | χ2 = 0.143, p = 0.705 |
| Marital Status (% Single) | 100% (n = 20) | 95% (n = 19) | χ2 = 2.105, p = 0.35 |
| Race/Ethnicity | |||
| Black/African American | 50% (n = 10) | 45% (n = 9) | χ2 = 1.26, p = 0.74 |
| Hispanic/Latino | 30% (n = 6) | 25% (n = 5) | |
| White | 20% (n = 4) | 25% (n = 5) | |
| Other | 0% (n = 0) | 5% (n = 1) | |
| Employment | |||
| Full-time | 45% (n = 9) | 25% (n = 5) | χ2 = 6.56, p = 0.161 |
| Part-time | 30% (n = 6) | 50% (n = 10) | |
| Student | 15% (n = 3) | 25% (n = 5) | |
| Unemployed | 10% (n = 2) | 0% | |
| Cannabis Use | |||
| Past week use of cannabis (# of days) | 6.4 (1.85) | N/A | N/A |
| Past 90 days use of cannabis (# of joints) | 246.1 (183.35) | N/A | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevorykin, A.; Ruglass, L.M.; Melara, R.D. Frontal Alpha Asymmetry and Inhibitory Control among Individuals with Cannabis Use Disorders. Brain Sci. 2019, 9, 219. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci9090219
Shevorykin A, Ruglass LM, Melara RD. Frontal Alpha Asymmetry and Inhibitory Control among Individuals with Cannabis Use Disorders. Brain Sciences. 2019; 9(9):219. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci9090219
Chicago/Turabian StyleShevorykin, Alina, Lesia M. Ruglass, and Robert D. Melara. 2019. "Frontal Alpha Asymmetry and Inhibitory Control among Individuals with Cannabis Use Disorders" Brain Sciences 9, no. 9: 219. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci9090219





