(−)-Epicatechin—An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
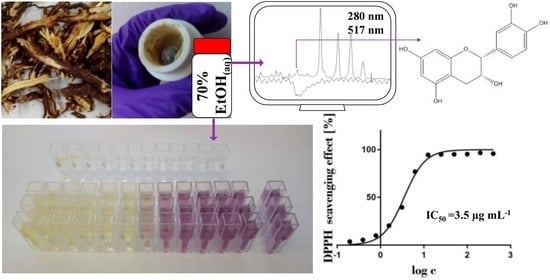
2.2. The Preparation, Extraction Yield, and Antioxidant Activity of Various Extracts
2.3. Comparison between the Antioxidant Activities of the 70% Ethanol(aq) Extract of Japanese Knotweed Rhizome Bark and Ascorbic Acid over Time
2.4. SEC-HPLC-UV Fractionation of the 70% Ethanol(aq) JKRB Extract Guided by an On-Line Post-Column Reaction with DPPH
2.5. Analyses of SEC Fractions and Determination of the Strongest Antioxidant by RP-HPLC-MS
2.6. Identification of the Compounds in the Antioxidant Fraction by Orthogonal Methods and Confirmation of Their Antioxidant Activity by DPPH Assay
3. Results and Discussion
3.1. Extraction Yields and Antioxidant Activity of Various Extracts
3.2. Antioxidant Activity over Time—Ascorbic Acid Compared to the JKRB 70% Ethanol(aq) Extract
3.3. SEC-HPLC Fractionation of the JKRB 70% Ethanol(aq) Extract, On-Line Post-Column Reaction of the SEC Fractions with DPPH and Determination of the Antioxidant Fractions
3.4. Characterization of the Compounds in the Isolated SEC Fractions, Identification of the Antioxidant Fraction Compounds by Orthogonal Methods, and their Antioxidant Activity over Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balogh, L. Japanese, Giant and Bohemian knotweed. In The Most Important Invasive Plants in Hungary; Botta-Dukat, Z., Balogh, L., Eds.; HAS Institute of Ecology and Botany: Budapest, Hungary, 2008; pp. 13–33. [Google Scholar]
- Zhang, H.; Li, C.; Kwok, S.-T.; Zhang, Q.-W.; Chan, S.-W. A review of the pharmacological effects of the dried root of Polygonum cuspidatum (Hu Zhang) and its constituents. Evid. Based Complementary Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-W.; Yang, F.-J.; Chen, C.-L.; Lee, W.-T.; Chen, R.-S. Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. J. Nat. Med. 2010, 64, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Chan, Y.-P.; Chang, J. Antioxidant activity of extract from Polygonum cuspidatum. Biol. Res. 2007, 40, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogačnik, L.; Rogelj, A.; Ulrih, N.P. Chemiluminescence method for evaluation of antioxidant capacities of different invasive knotweed species. Anal. Lett. 2015, 49, 350–363. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmianski, J. Profile of bioactive compounds in the morphological parts of wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and their antioxidative activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef] [Green Version]
- Ardelean, F.; Moacă, E.A.; Păcurariu, C.; Antal, D.S.; Dehelean, C.; Toma, C.-C.; Drăgan, S. Invasive Polygonum cuspidatum: Physico-chemical analysis of a plant extract with pharmaceutical potential. Stud. Univ. Vasile Goldis Arad Seria Stiintele Vietii 2016, 26, 415–421. [Google Scholar]
- Kurita, S.; Kashiwagi, T.; Ebisu, T.; Shimamura, T.; Ukeda, H. Content of resveratrol and glycoside and its contribution to the antioxidative capacity of Polygonum cuspidatum (Itadori) harvested in Kochi. Biosci. Biotechnol. Biochem. 2014, 78, 499–502. [Google Scholar] [CrossRef]
- Chan, C.-L.; Gan, R.-Y.; Corke, H. The phenolic composition and antioxidant capacity of soluble and bound extracts in selected dietary spices and medicinal herbs. Int. J. Food Sci. Technol. 2016, 51, 565–573. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Ślusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical diversity in rhizomes of three Reynoutria species and their antioxidant activity correlations elucidated by LC-ESI-MS/MS analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef] [Green Version]
- Pogačnik, L.; Bergant, T.; Skrt, M.; Poklar Ulrih, N.; Viktorová, J.; Ruml, T. In vitro comparison of the bioactivities of Japanese and Bohemian knotweed ethanol extracts. Foods 2020, 9, 544. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Zhang, Y.; Xu, Q.; Xiao, H.; Liang, X. Analysis of estrogenic compounds in Polygonum cuspidatum by bioassay and high performance liquid chromatography. J. Ethnopharmacol. 2006, 105, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008, 109, 530–537. [Google Scholar] [CrossRef]
- Lin, H.-W.; Sun, M.-X.; Wang, Y.-H.; Yang, L.-M.; Yang, Y.-R.; Huang, N.; Xuan, L.-J.; Xu, Y.-M.; Bai, D.-L.; Zheng, Y.-T.; et al. Anti-HIV activities of the compounds isolated from Polygonum cuspidatum and Polygonum multiflorum. Planta Med. 2010, 76, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, P.; Zhang, T.; Hostettmann, K. Anti-inflammatory activity of the invasive neophyte Polygonum cuspidatum Sieb. and Zucc. (Polygonaceae) and the chemical comparison of the invasive and native varieties with regard to resveratrol. J. Tradit. Complement. Med. 2013, 3, 182–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Liang, J. Screening of bioactive compounds in rhizoma Polygoni cuspidati with hepatocyte membranes by HPLC and LC-MS. J. Sep. Sci. 2014, 37, 250–256. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Vanden Berge, D.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Bristi, N.J.; Rifiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. 2011, 1. [Google Scholar] [CrossRef] [Green Version]
- Ojha, K.; Dubey, S.; Chandrakar, J.; Minj, R.A.; Dehariya, R.; Dixit, A.K. A review on different methods of determination of antioxidant activity assay of herbal plants. Res. J. Life Sci. Bioinf. Pharm. Chem. Sci. 2018, 4, 707–730. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Vovk, I.; Simonovska, B.; Andrenšek, S.; Vuorela, H.; Vuorela, P. Rotation planar extraction and rotation planar chromatography of oak (Quercus robur L.) bark. J. Chromatogr. A 2003, 991, 267–274. [Google Scholar] [CrossRef]
- Orsini, F.; Vovk, I.; Glavnik, V.; Jug, U.; Corradini, D. HPTLC, HPTLC-MS/MS and HPTLC-DPPH methods for analyses of flavonoids and their antioxidant activity in Cyclanthera pedata leaves, fruits and dietary supplement. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 290–301. [Google Scholar] [CrossRef]
- Simonovska, B.; Vovk, I.; Andrenšek, S.; Valentova, K.; Ulrichová, J. Investigation of phenolic acids in yacon (Smallanthus sonchifolius) leaves and tubers. J. Chromatogr. A 2003, 1016, 89–98. [Google Scholar] [CrossRef]
- Cieśla, Ł.; Kryszeń, J.; Stochmal, A.; Oleszek, W.; Waksmundzka-Hajnos, M. Approach to develop a standardized TLC-DPPH test for assessing free radical scavenging properties of selected phenolic compounds. J. Pharm. Biomed. Anal. 2012, 70, 126–135. [Google Scholar] [CrossRef]
- Meda, N.R.; Fraisse, D.; Gnoula, C.; Vivier, M.; Felgines, C.; Senejoux, F. Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. Eur. Food Res. Technol. 2017, 243, 1659–1666. [Google Scholar] [CrossRef]
- Wu, J.-H.; Huang, C.-Y.; Tung, Y.-T.; Chang, S.-T. On-line RP-HPLC-DPPH screening method for detection of radical-scavenging phytochemicals from flowers of Acacia confuse. J. Agric. Food Chem. 2008, 56, 328–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Xing, H.; Lu, X.; Zhao, L.; Qu, K.; Bi, K. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC-DPPH assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Koleva, I.I.; Niederländer, H.A.G.; Van Beek, T.A. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal. Chem. 2000, 72, 2323–2328. [Google Scholar] [CrossRef]
- Bandoniene, D.; Murkovic, M. On-line HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from apples (Malus domestica L.). J. Agric. Food Chem. 2002, 50, 2482–2487. [Google Scholar] [CrossRef]
- Burnaz, N.A.; Küçük, M.; Akar, Z. An on-line HPLC system for detection of antioxidant compounds in some plant extracts by comparing three different methods. J. Chromatogr. B 2017, 1052, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pravadali-Cekic, S.; Kocic, D.; Hua, S.; Jones, A.; Dennis, G.R.; Shalliker, R.A. Tuning a parallel segmented flow column and enabling multiplexed detection. J. Visualized Exp. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma Avasthi, A.; Bhatnagar, M.; Sarkar, N.; Kitchlu, S.; Ghosal, S. Bioassay guided screening, optimization and characterization of antioxidant compounds from high altitude wild edible plants of Ladakh. J. Food Sci. Technol. 2016, 53, 3244–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudha, A.; Srinivasan, P. Bioassay-guided isolation and antioxidant evaluation of flavonoid compound from aerial parts of Lippia nodiflora L. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jothy, S.L.; Saito, T.; Kanwar, J.R.; Kavitha, S.; Herng, L.C.; Chen, Y.; Yin-Hui, L.; Sasidharan, S. Bioassay-guided isolation and antioxidant evaluation of rutin from leaf of Polyalthia longifolia. Asian J. Appl. Sci. 2017, 5, 138–148. [Google Scholar]
- Lin, H.-Y.; Kuo, Y.-H.; Lin, Y.-L.; Chiang, W. Antioxidative effect and active components from leaves of lotus (Nelumbo nucifera). J. Agric. Food Chem. 2009, 57, 6623–6629. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Chai, H.-B.; Keller, W.J.; Douglas Kinghorn, A. Lignans and other constituents of the fruits of Euterpe oleracea (Açai) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Sunil, J.; Janapati, Y.K.; Bramhachari, P.V. Bioassay guided isolation and identification of the antioxidant constituent from Holostemma ada-kodien shcult. Int. J. Pharma Bio Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Lelono, R.A.A.; Tachibana, S. Bioassay-guided isolation and identification of antioxidative compounds from the bark of Eugenia polyantha. Pak. J. Biol. Sci. 2013, 16, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wang, H.; Li, Y.; He, T.; Wang, D.; Wang, W.; Jia, W.; Lin, Z.; Chen, S. One injection to profile the chemical composition and dual-antioxidation activities of Rosa chinensis Jacq. J. Chromatogr. A 2000, 1613. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yue, W.; Li, Q. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhang, H.; Cai, Z. Analysis of rhizoma Polygoni cuspidati by HPLC and HPLC-ESI/MS. Phytochem. Anal. 2007, 18, 387–392. [Google Scholar] [CrossRef]
- Fan, P.; Hay, A.-E.; Marston, A.; Lou, H.; Hostettmann, K. Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. and Zucc and Polygonum sachalinensis F. Schmidt ex Maxim. Biochem. Syst. Ecol. 2009, 37, 24–34. [Google Scholar] [CrossRef]
- Beňová, B.; Adam, M.; Pavlíková, P.; Fischer, J. Supercritical fluid extraction of piceid, resveratrol and emodin from Japanese knotweed. J. Supercrit. Fluids 2010, 51, 325–330. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.X.; Kongstad, K.T.; Jäger, A.K.; Staerk, D. Potential of Polygonum cuspidatum root as an antidiabetic food: Dual high-resolution α-glucosidase and PTP1B inhibition profiling combined with HPLC-HRMS and NMR for identification of antidiabetic constituents. J. Agric. Food. Chem. 2017, 65, 4421–4427. [Google Scholar] [CrossRef]
- Fu, J.; Wang, M.; Guo, H.; Tian, Y.; Zhang, Z.; Song, R. Profiling of components of rhizoma et radix Polygoni cuspidati by high-performance liquid chromatography with ultraviolet diode-array detector and ion trap/time-of-flight mass spectrometric detection. Pharmacogn. Mag. 2015, 11, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Nawrot-Hadzik, I.; Granica, S.; Domaradzki, K.; Pecio, Ł.; Matkowski, A. Isolation and determination of phenolic glycosides and anthraquinones from rhizomes of various Reynoutria species. Planta Med. 2018, 84, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Lachowicz, S.; Oszmiański, J.; Wojdyło, A.; Cebulak, T.; Hirnle, L.; Siewiński, M. UPLC-PDA-Q/TOF-MS identification of bioactive compounds and on-line UPLC-ABTS assay in Fallopia japonica Houtt and Fallopia sachalinensis (F. Schmidt) leaves and rhizomes grown in Poland. Eur. Food. Res. Technol. 2019, 245, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Glavnik, V.; Vovk, I.; Albreht, A. High performance thin-layer chromatography-mass spectrometry of Japanese knotweed flavan-3-ols and proanthocyanidins on silica gel plates. J. Chromatogr. A 2017, 1482, 97–108. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I. High performance thin-layer chromatography-mass spectrometry methods on diol stationary phase for the analyses of flavan-3-ols and proanthocyanidins in invasive Japanese knotweed. J. Chromatogr. A 2019, 1598, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.-Z.; Liu, S.; Zhou, L.-L. Rapid quantitative HPTLC analysis, on one plate, of emodin, resveratrol, and polydatin in the Chinese herb Polygonum cuspidatum. Chromatographia 2005, 61, 311–314. [Google Scholar] [CrossRef]
- Hawrył, M.A.; Waksmundzka-Hajnos, M. Two-dimensional thin-layer chromatography of selected Polygonum sp. extracts on polar-bonded stationary phases. J. Chromatogr. A 2011, 1218, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Vaher, M.; Koel, M. Separation of polyphenolic compounds extracted from plant matrices using capillary electrophoresis. J. Chromatogr. A 2003, 990, 225–230. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kawanishi, K.; Tagahara, K.; Kobayashi, N. Capillary electrophoresis for simultaneous determination of emodin, chrysophanol, and their 8-β-D-glucosides. Chem. Pharm. Bull. 2003, 51, 418–420. [Google Scholar] [CrossRef] [Green Version]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Melo, P.S.; Massarioli, A.P.; Moreno, I.A.M.; Bezerra, R.M.N.; Rosalen, P.L.; Da Silva, G.V.J.; Nascimento, A.M.; Alencar, S.M. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. 2016, 192, 306–312. [Google Scholar] [CrossRef] [Green Version]
- GraphPad Prism; Version 700 for Windows; GraphPad Software: La Jolla/San Diego, CA, USA, 2016.
- Glavnik, V.; Simonovska, B.; Vovk, I. Densitometric determination of (+)-catechin and (-)-epicatechin by 4-dimethylaminocinnamaldehyde reagent. J. Chromatogr. A 2009, 1216, 4485–4491. [Google Scholar] [CrossRef]
- Vovk, I.; Simonovska, B.; Vuorela, H. Separation of eight selected flavan-3-ols on cellulose thin-layer chromatographic plates. J. Chromatogr. A 2005, 1077, 188–194. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I. Analysis of dietary supplements. In Instrumental Thin-Layer Chromatography; Poole, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 589–635. [Google Scholar]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kurechi, T.; Kikugawa, K.; Kato, T. Studies on the antioxidants. XIII. Hydrogen donating capability of antioxidants to 2,2-diphenyl-1-picrylhydrazyl. Chem. Pharm. Bull. 1980, 28, 2089–2093. [Google Scholar] [CrossRef] [Green Version]
- Jug, U.; Glavnik, V.; Vovk, I.; Makuc, D.; Naumoska, K. Off-line multidimensional high performance thin-layer chromatography for fractionation of Japanese knotweed rhizome bark extract and isolation of flavan-3-ols, proanthocyanidins and anthraquinones. J. Chromatogr. A 2021, 1637, 461802. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. Available online: https://www.ptfarm.pl/wydawnictwa/czasopisma/acta-poloniae-pharmaceutica/110/-/12992 (accessed on 10 September 2020).
- Wawrzyniak, J.; Ryniecki, A.; Zembrzuski, W. Application of voltammetry to determine vitamin C in apple juices. Acta Sci. Pol. Technol. Aliment. 2005, 4, 5–16. [Google Scholar]
- Adepoju, T.S.; Olasehinde, E.F.; Aderibigbe, A.D. Effect of sodium metabisulphite and disodium ethylenediaminetetraacetic acid (EDTA) on the stability of ascorbic acid in vitamin C syrup. Researcher 2014, 6, 6–9. [Google Scholar]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Matkowski, A.; Jamiołkowska-Kozlowska, W.; Nawrot, I. Chinese medicinal herbs as source of antioxidant compounds-where tradition meets the future. Curr. Med. Chem. 2013, 20, 984–1004. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I. Extraction of anthraquinones from Japanese knotweed rhizomes and their analyses by high performance thin-layer chromatography and mass spectrometry. Plants 2020, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- Ha Lai, T.N.; Herent, M.-F.; Quetin-Leclercq, J.; Thuy Nguyen, T.B.; Rogez, H.; Larondelle, Y.; André, C.M. Piceatannol, a potent bioactive stilbene, as major phenolic component in Rhodomyrtus tomentosa. Food Chem. 2013, 138, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Kranl, K.; Schlesier, K.; Bitsch, R.; Hermann, H.; Rohe, M.; Böhm, V. Comparing antioxidative food additives and secondary plant products—Use of different assays. Food Chem. 2005, 93, 171–175. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragrance J. 2010, 25, 291–312. [Google Scholar] [CrossRef]










| Extraction Solvents | ||||||||
|---|---|---|---|---|---|---|---|---|
| Water | Methanol | Acetone | Ethanol | Ethyl Acetate | ||||
| 100% | 80% (aq) | 100% | 70% (aq) | 100% | 70% (aq) | 90% (aq) | ||
| Extraction yield (w/w %) | 25.8 | 38.1 | 37.2 | 21.1 | 42.6 | 29.3 | 44.3 | 14.9 |
| IC50 (µg mL−1) | 3.561 | 3.715 | 3.469 | 2.632 | 3.350 | 2.893 | 3.503 | 2.786 |
| LogIC50 | 0.552 | 0.570 | 0.540 | 0.420 | 0.525 | 0.461 | 0.544 | 0.445 |
| LogIC50 std. error | 0.016 | 0.014 | 0.022 | 0.018 | 0.017 | 0.016 | 0.014 | 0.020 |
| Hillslope | 1.607 | 1.884 | 1.669 | 1.911 | 1.665 | 1.924 | 1.789 | 1.756 |
| Hillslope std. error | 0.083 | 0.105 | 0.125 | 0.136 | 0.097 | 0.123 | 0.093 | 0.124 |
| 0 h | 2 h | 4 h | 6 h | 8 h | 24 h | 50 h | 7 d | 14 d | |
|---|---|---|---|---|---|---|---|---|---|
| IC50 AA (µM) | 17.6853.1 | 30.524 | 37.662 | 45.846 | 56.612 | ~96.886 | 164.933 | ~219.382 | 356.495 |
| IC50 AA (µg mL−1) | 15 | 5.376 | 6.633 | 8.075 | 9.971 | ~17.064 | 29.049 | ~38.637 | 62.787 |
| LogIC50 (µM) | 1.248 | 1.485 | 1.576 | 1.661 | 1.753 | ~1.986 | 2.217 | ~2.341 | 2.552 |
| LogIC50 std. error | 0.011 | 0.005 | 0.003 | 0.006 | 0.006 | ~1.293 | 0.020 | ~33.874 | 0.015 |
| Hillslope | 2.606 | 5.344 | 6.492 | 6.060 | 5.673 | ~17.640 | 7.444 | ~16.268 | 9.400 |
| Hillslope std. error | 0.149 | 0.304 | 0.254 | 0.566 | 0.383 | ~1659.493 | 0.771 | ~9712.611 | 0.938 |
| IC50 JKRB (µg mL−1) | 3.503 | 3.684 | 3.662 | 3.876 | 3.947 | 3.530 | 3.759 | 3.731 | 3.325 |
| LogIC50 | 0.544 | 0.566 | 0.564 | 0.588 | 0.596 | 0.548 | 0.575 | 0.572 | 0.522 |
| LogIC50 std. error | 0.014 | 0.013 | 0.015 | 0.017 | 0.021 | 0.016 | 0.011 | 0.014 | 0.024 |
| Hillslope | 1.789 | 1.815 | 1.858 | 1.796 | 1.736 | 2.044 | 1.933 | 1.737 | 1.531 |
| Hillslope std. error | 0.093 | 0.086 | 0.106 | 0.117 | 0.128 | 0.140 | 0.085 | 0.088 | 0.118 |
| FR | tR a [min] | MS [M-H]− | MS2 and MS3 b | Tentatively Identified Compounds |
|---|---|---|---|---|
| 1 | 6.9 | 395 | [395]: 215 | c |
| 10.2, 9.4 | 1005 | [1005]: 713, 917, 961, 458 | derivative of emodin bianthrone-hexose-malonic acid [10] | |
| 2 | 7.3 | 521 | [521]: 359 | c |
| 7.3 | 581 | [581]: 521, 522, 544, 563, 499, 483, 417 | c | |
| 7.3 | 603 | [603]: 543, 521 | c | |
| 3 | 10.2 | 919 | [919]: 713, 671, 875, 458, 509, 416 | emodin bianthrone-hexose-(malonic acid)-hexose [10] |
| 10.2 | 941 | No data | c | |
| 11.4, 12.2 | 933 | [933]: 889, 458, 727 | methyl derivative of emodin bianthrone-hexose-(malonic acid)-hexose [10] | |
| 4 | 10.2 | 919 | [919]: 713, 671, 875, 458, 416, 509 | emodin bianthrone-hexose-(malonic acid)-hexose [10] |
| 10.2 | 1005 | [1005]: 917, 961, 875, 713, 458 | derivative of emodin bianthrone-hexose-malonic acid [10] | |
| 10.2, 12.0 | 1027 | [1027]: 939, 983, 735 (10.2 min) | c | |
| [1027]: 389, 489, 533, 449, 744, 862, 939, 983, | c | |||
| 994 (12.0 min) | ||||
| 12.3 | 1009 | [1009]: 471, 389, 515, 921, 965 | c | |
| 12.3 | 987 | [987]: 449, 943 | c | |
| 12.3 | 449 | [449]: 245 | torachrysone 8-O-(6′-O-acetyl)-glucoside [67] | |
| 5 | 6.6, 10.6 | 473 | [473]: 455, 413 (6.6 min) | c |
| [473]: 269 (10.6 min) | emodin-O-(acetyl)-hexoside [67] | |||
| 6.6, 10.6 | 605 | [605]: 587 | c | |
| 6.6, 10.6 | 665 | [665]: 647, 605, 589, 545, 501, 567 | c | |
| 10.6, 20.6 | 269 | [269]: 225, 269, 251, 241, 187 | emodin [67,74] | |
| 6 | 11.2 | 265 | No data | c |
| 11.2 | 297 | No data | c | |
| 11.2, 11.8 | 1005, 502, 458 | [1005]: 713, 917, 458 | derivative of emodin bianthrone-hexose-malonic acid [10] | |
| 11.2, 11.8 | 1027 | [1027]: 781, 863, 699, 715, 945, 617 | c | |
| 13.7 | 1019, 975 | [1019]: 691, 773, 855, 609, 527, 937 | derivative of bianthrone [10] | |
| 11.2 | 265 | No data | c | |
| 7 | 11.6 | 407 | [407]: 245 | torachrysone-8-O-glucoside/procyanidin degradation product [67] |
| 13.5 | 933 | [933]: 889, 685, 416 | methyl derivative of emodin bianthrone-hexose-(malonic acid)-hexose [10] | |
| 13.5 | 1019 | No data | c | |
| 8 | 5.6, 6.3 | 289 | [289]: 245, 205, 179, 203; | (−)-epicatechin (6.32 min) [67], (−)-epicatechin standard |
| [289➔245] b: 203, 227, 161, 175, 187, 217, 245 | ||||
| 9.2, 8.3 | 431 | [431]: 227, 389 | resveratrol acetyl hexoside [67] | |
| 9.7, 9.0, 8.3 | 445 | [445]: 385 (9.7 min) | c | |
| [445]: 281, 325, 369, 427, 263, 211 (9.0, 8.3 min) | c | |||
| 9.2, 8.3 | 475 | [475]: 431 | resveratrol malonyl hexoside [67] | |
| 9.2, 8.3 | 491 | [491]: 431 | c | |
| 9.2, 8.3 | 227 | [227]: 185, 183, 159, 157, 227, 209, 143 | resveratrol [67,52] | |
| 5.6, 6.3 | 289 | [289]: 245, 205, 179, 203; | (−)-epicatechin (6.32 min) [67], (−)-epicatechin standard | |
| 9 | 5.6 | 289 | [289]: 245, 205, 203, 179 | catechin [67] |
| 9.9 | 431 | [431]: 269 | emodin-O-hexoside [67] | |
| 9.9, 20.5 | 269 | [269]: 269, 225, 241, 251, 209, 271 | emodin [67,74] | |
| 9.9 | 385 | No data | c | |
| 10 | 7.1, 8.9 | 389 | [389]: 227 | polydatin (piceid)/resveratroloside [67] |
| 7.1, 8.9 | 425d | [425]: 389 | polydatin (piceid) (dihydrate)/resveratroloside (dihydrate) [67] | |
| 7.1 | 449d | [449]: 389, 227 | resveratrol acetyl hexoside (hydrate) [67] | |
| 7.1, 8.9 | 227 | [227]: 185, 183, 159, 157, 209, 143, 165 | resveratrol [67] | |
| 10.8 | 473 | [473]: 269, 311 | emodin-O-(acetyl)-hexoside [67] | |
| 10.8 | 517 | [517]: 473, 431 | emodin-O-(6′-O-malonyl)-hexoside [67] | |
| 11 | 6.6, 8.3 | 405 | [405]: 243 | piceatannol-3-O-glucoside [10] |
| 6.6, 8.3 | 243 | [243]: 225, 201, 199, 175, 215, 159 | piceatannol [75] | |
| 12 | 7.9, 9.1 | 389 | [389]: 227 | polydatin (piceid)/resveratroloside [67] |
| 7.9, 9.1 | 227 | [227]: 185, 183, 209, 159, 157, 165, 143 | resveratrol [67] | |
| 7.9, 9.1 | 425 d | [425]: 389, 227 | polydatin (piceid) (dihydrate)/resveratroloside (dihydrate) [67] | |
| 13 | 11.6 | 431 | [431]: 269, 311, 413 | emodin-O-hexoside [67] |
| 11.6 | 269 | [269]: 225, 269, 241, 251 | emodin [67,74] | |
| 4.9 | 565 | No data | c | |
| 14 | 7.2, 9.5 | 245 | [245]: 230 (7.2 min) | c [67] |
| [245]: 229 (230) (9.5 min) | c [67] | |||
| 7.2, 9.5 | 325 d | [325]: 245 (7.2 min) | catechin dihydrate/unknown [67] | |
| 7.2, 9.5 | 245 | [325]: 244 (245), 203, 283 (9.5 min) | catechin dihydrate/unknown [67] |
| 0 h | 2 h | 4 h | 6 h | 8 h | 24 h | 50 h | 7 d | 14 d | |
|---|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | 6.298 | 4.967 | 4.738 | 5.539 | 6.393 | 4.949 | 5.129 | 6.280 | 4.190 |
| IC50 (µg mL−1) | 1.828 | 1.442 | 1.375 | 1.608 | 1.856 | 1.436 | 1.489 | 1.823 | 1.216 |
| LogIC50 | 0.799 | 0.696 | 0.676 | 0.744 | 0.806 | 0.695 | 0.710 | 0.798 | 0.622 |
| LogIC50 std. error | 0.039 | 0.040 | 0.047 | 0.045 | 0.043 | 0.044 | 0.041 | 0.052 | 0.039 |
| Hillslope | 1.690 | 1.427 | 1.282 | 1.441 | 1.669 | 1.315 | 1.367 | 1.375 | 1.538 |
| Hillslope std. error | 0.176 | 0.110 | 0.106 | 0.134 | 0.188 | 0.107 | 0.109 | 0.153 | 0.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jug, U.; Naumoska, K.; Vovk, I. (−)-Epicatechin—An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation. Antioxidants 2021, 10, 133. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10010133
Jug U, Naumoska K, Vovk I. (−)-Epicatechin—An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation. Antioxidants. 2021; 10(1):133. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10010133
Chicago/Turabian StyleJug, Urška, Katerina Naumoska, and Irena Vovk. 2021. "(−)-Epicatechin—An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation" Antioxidants 10, no. 1: 133. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10010133