Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Methods
2.3.1. Bread Preparation
2.3.2. Antioxidant Content and Antioxidant Activity
2.3.3. Determination of Individual Polyphenols by UPLC-PDA-MS/MS
- Extraction
- 2.
- Assay
2.3.4. Organoleptic Analysis
2.3.5. Statistical Analysis
3. Results and Discussion
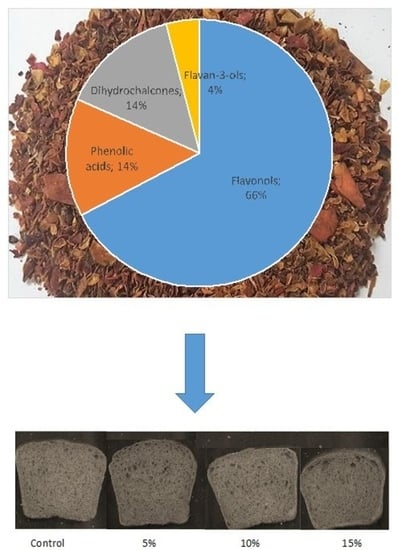
3.1. Apple Pomace Characteristics
3.2. Profile of Phenolic Compounds in Gluten-Free Bread Enriched with Apple Pomace
3.3. Organoleptic Analysis of Gluten-Free Bread Enriched with Apple Pomace
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Reguła, J.; Suliburska, J.; Złotek, U.; Gawlik-Dziki, U. Effects of Gluten-Free Breads, with Varying Functional Supplements, on the Biochemical Parameters and Antioxidant Status of Rat Serum. Food Chem. 2015, 182, 268–274. [Google Scholar] [CrossRef]
- Tsatsaragkou, Κ.; Protonotariou, S.; Mandala, I. Structural Role of Fibre Addition to Increase Knowledge of Non-Gluten Bread. J. Cereal Sci. 2016, 67, 58–67. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The Gluten-Free Diet: Safety and Nutritional Quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Hopper, A.D.; Hadjivassiliou, M.; Butt, S.; Sanders, D.S. Adult Coeliac Disease. BMJ 2007, 335, 558–562. [Google Scholar] [CrossRef]
- Wierdsma, N.; Bokhorst-de van der Schueren, M.; Berkenpas, M.; Mulder, C.; van Bodegraven, A. Vitamin and Mineral Deficiencies Are Highly Prevalent in Newly Diagnosed Celiac Disease Patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef] [PubMed]
- Stojiljković, V.; Todorović, A.; Pejić, S.; Kasapović, J.; Saičić, Z.S.; Radlović, N.; Pajović, S.B. Antioxidant Status and Lipid Peroxidation in Small Intestinal Mucosa of Children with Celiac Disease. Clin. Biochem. 2009, 42, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Szaflarska-Popławska, A.; Siomek, A.; Czerwionka-Szaflarska, M.; Gackowski, D.; Różalski, R.; Guz, J.; Szpila, A.; Zarakowska, E.; Oliński, R. Oxidatively Damaged DNA/Oxidative Stress in Children with Celiac Disease. Cancer Epidemiol. Biomark. Prev 2010, 19, 1960–1965. [Google Scholar] [CrossRef] [Green Version]
- Maluf, S.W.; Wilhelm Filho, D.; Parisotto, E.B.; Medeiros, G.d.S.d.; Pereira, C.H.J.; Maraslis, F.T.; Dornelles Schoeller, C.C.; da Rosa, J.S.; Fröde, T.S.; Maluf, S.W.; et al. DNA Damage, Oxidative Stress, and Inflammation in Children with Celiac Disease. Genet. Mol. Biol. 2020, 43. [Google Scholar] [CrossRef]
- Rowicka, G.; Czaja-Bulsa, G.; Chełchowska, M.; Riahi, A.; Strucińska, M.; Weker, H.; Ambroszkiewicz, J. Oxidative and Antioxidative Status of Children with Celiac Disease Treated with a Gluten Free-Diet. Available online: https://www.hindawi.com/journals/omcl/2018/1324820/ (accessed on 22 April 2021).
- Tarko, T.; Duda-Chodak, A.; Bebak, A. Aktywność biologiczna wybranych wytłoków owocowych oraz warzywnych. Żywność Nauka Technol. Jakość 2012, 19, 4. [Google Scholar]
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive Properties of Flavonoid-Rich Apple Peel Extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute Anti-Hyperglycaemic Effects of an Unripe Apple Preparation Containing Phlorizin in Healthy Volunteers: A Preliminary Study. J. Sci. Food Agric. 2015, 95, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of Polyphenolic Content and in Vitro Antiradical Characteristics of Apple Pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of Apple By-Products as Source of New Ingredients: Current Situation and Perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of Polyphenols from the By-Products of Plant Food Processing and Application as Valuable Food Ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; de Jesús Zazueta-Morales, J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; de Jesús Ruiz-Anchondo, T. Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace in an Extruded Cereal. LWT Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of Apple Pomace on Quality Characteristics of Brown Rice Based Cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of Raspberry and Blueberry Pomace through the Formulation of Value-Added Gluten-Free Cookies. J. Food Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlatanović, S.; Kalušević, A.; Micić, D.; Laličić-Petronijević, J.; Tomić, N.; Ostojić, S.; Gorjanović, S. Functionality and Storability of Cookies Fortified at the Industrial Scale with up to 75% of Apple Pomace Flour Produced by Dehydration. Foods 2019, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F.J.P.H.; van Buul, V.J.; Shewry, P.R. Does Wheat Make Us Fat and Sick? J. Cereal Sci. 2013, 58, 209–215. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Ziobro, R.; Kruczek, M. Enrichment of Wheat Bread with Apple Pomace as a Way to Increase Pro-Health Constituents. Qual. Assur. Saf. Crops Foods 2019, 11, 231–240. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of Flavonoids in the Resistance of Two Poplar Cultivars to Mistletoe (Viscum Album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Application of Ultra Performance Liquid Chromatography-Photodiode Detector-Quadrupole/Time of Flight-Mass Spectrometry (UPLC-PDA-Q/TOF-MS) Method for the Characterization of Phenolic Compounds of Lepidium Sativum L. Sprouts. Eur. Food Res. Technol. 2013, 236, 699–706. [Google Scholar] [CrossRef] [Green Version]
- PN-ISO 8589. Sensory Analysis—General Guidance for the Design of Test Rooms; PKN: Warsaw, Poland, 1998; (In Polish, English Abstract). [Google Scholar]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Optimisation of the Phenolic Content and Antioxidant Activity of Apple Pomace Aqueous Extracts. CyTA J. Food 2015, 13, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.-L.; Yue, T.-L.; Yuan, Y.-H.; Zhang, H.-W. Optimization of Microwave-Assisted Extraction of Polyphenols from Apple Pomace Using Response Surface Methodology and HPLC Analysis: Sample Preparation. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Adil, İ.H.; Çetin, H.I.; Yener, M.E.; Bayındırlı, A. Subcritical (Carbon Dioxide+ethanol) Extraction of Polyphenols from Apple and Peach Pomaces, and Determination of the Antioxidant Activities of the Extracts. J. Supercrit. Fluids 2007, 43, 55–63. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical Composition of Apple Fruit, Juice and Pomace and the Correlation between Phenolic Content, Enzymatic Activity and Browning. LWT Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Krasnova, I.; Segliņa, D. Content of Phenolic Compounds and Antioxidant Activity in Fresh Apple, Pomace and Pomace Water Extract—Effect of Cultivar. Proc. Latv. Acad. Sci. 2019, 73, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional Properties, Phenolic Constituents and Antioxidant Potential of Industrial Apple Pomace for Utilization as Active Food Ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.F.; Sanchez-Reus, I.; Iglesias, I.; Benedi, J. Quercetin, a Flavonoid Antioxidant, Prevents and Protects against Ethanol-Induced Oxidative Stress in Mouse Liver. Biol. Pharm. Bull. 2003, 26, 1398–1402. [Google Scholar] [CrossRef] [Green Version]
- Faillie, J.-L. Pharmacological Aspects of the Safety of Gliflozins. Pharmacol. Res. 2017, 118, 71–81. [Google Scholar] [CrossRef]
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of Chlorogenic Acid in Vegetables and Fruits as an Inhibitor of 8-Hydroxydeoxyguanosine Formation in Vitro and in a Rat Carcinogenesis Model. Food Chem. Toxicol. 2000, 38, 467–471. [Google Scholar] [CrossRef]
- Peng, I.-W.; Kuo, S.-M. Flavonoid Structure Affects the Inhibition of Lipid Peroxidation in Caco-2 Intestinal Cells at Physiological Concentrations. J. Nutr. 2003, 133, 2184–2187. [Google Scholar] [CrossRef] [Green Version]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Pieszka, M.; Szczurek, P.; Bederska-Łojewska, D.; Migdał, W.; Pieszka, M.; Gogol, P.; Jagusiak, W. The Effect of Dietary Supplementation with Dried Fruit and Vegetable Pomaces on Production Parameters and Meat Quality in Fattening Pigs. Meat Sci. 2017, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects and Applications; Technomic Publishing Company: Lancaster, UK, 1995; ISBN 9781566762793. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin−Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.-H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran Fermentation as a Means to Enhance Technological Properties and Bioactivity of Rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef]
- Korus, J.; Juszczak, L.; Ziobro, R.; Witczak, M.; Grzelak, K.; Sójka, M. Defatted Strawberry and Blackcurrant Seeds as Functional Ingredients of Gluten-Free Bread. J. Text. Stud. 2012, 43, 29–39. [Google Scholar] [CrossRef]
- Boskov Hansen, H.; Andreasen, M.; Nielsen, M.; Larsen, L.; Knudsen, B.K.; Meyer, A.; Christensen, L.; Hansen, Å. Changes in Dietary Fibre, Phenolic Acids and Activity of Endogenous Enzymes during Rye Bread-Making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Rupasinghe, H.; Wang, L.; Huber, G.; Pitts, N. Effect of Baking on Dietary Fibre and Phenolics of Muffins Incorporated with Apple Skin Powder. Food Chem. 2007, S0308814607009740. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of Antioxidant Activity during Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugas, B.; Dugas, N.; Conti, M.; Calenda, A.; Pino, P.; Thomas, Y.; Mazier, D.; Vouldoukis, I. Wheat Gliadin Promotes the Interleukin-4-Induced IgE Production by Normal Human Peripheral Mononuclear Cells through a Redox-Dependent Mechanism. Cytokine 2003, 21, 270–280. [Google Scholar] [CrossRef]
- Tučková, L.; Novotná, J.; Novák, P.; Flegelová, Z.; Květoň, T.; Jelínková, L.; Zídek, Z.; Man, P.; Tlaskalová-Hogenová, H. Activation of Macrophages by Gliadin Fragments: Isolation and Characterization of Active Peptide. J. Leukoc. Biol. 2002, 71, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and Antioxidant Composition of By-Products from the Cider Industry: Apple Pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Vinaykumar, N.M.; Mahmood, R.; Krishna, V.; Ravishankara, B.; Shastri, S.L. Antioxidant and in Vivo Hepatoprotective Effects of Gardenia Gummifera L.f. Fruit Methanol Extract. Clin. Phytosci. 2020, 6, 47. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From In Vitro Results to In Vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Bchir, B.; Rabetafika, H.N.; Paquot, M.; Blecker, C. Effect of Pear, Apple and Date Fibres from Cooked Fruit By-Products on Dough Performance and Bread Quality. Food Bioprocess. Technol. 2014, 7, 1114–1127. [Google Scholar] [CrossRef]
- Torbica, A.; Škrobota, D.; Janić Hajnal, E.; Belović, M.; Zhang, N. Sensory and Physico-Chemical Properties of Wholegrain Wheat Bread Prepared with Selected Food by-Products. LWT 2019, 114, 108414. [Google Scholar] [CrossRef]
- Rocha Parra, A.F.; Ribotta, P.D.; Ferrero, C. Apple Pomace in Gluten-Free Formulations: Effect on Rheology and Product Quality. Int. J. Food Sci. Technol. 2015, 50, 682–690. [Google Scholar] [CrossRef]
- Jannati, N.; Hojjatoleslamy, M.; Hosseini, E.; Mozafari, H.R.; Siavoshi, M. Effect of Apple Pomace Powder on Rheological Properties of Dough and Sangak Bread Texture. Carpathian J. Food Sci. Technol. 2018, 10, 77–84. [Google Scholar]

| By-Product | Total Phenolic Content (mg Gallic Acid/100 g d.m.) | Total Flavonoids Content (mg Rutin/100 g d.m.) | Trolox Equivalent Antioxidant Capacity (mg Tx/g d.m.) |
|---|---|---|---|
| Apple pomace (AP) | 89.4 | 94.3 | 9.30 |
| Compounds | Content in Apple Pomace (mg/100 g d.m.) | |
|---|---|---|
| Flavonols | luteolin 6-C-hexoside O-hexoside | n.d. |
| luteolin O-hexoside C-hexoside | n.d. | |
| quercetin-O-rutinoside | 2.82 ± 0.02 | |
| quercetin-3-O-galactoside | 22.55 ± 0.34 | |
| quercetin-3-O-glucoside | 5.88 ± 0.10 | |
| quercetin-3-O-arabinoside | 8.77 ± 0.27 | |
| quercetin-3-O-xyloside | 13.91 ± 0.03 | |
| quercetin-3-O-rhamnoside | 19.21 ± 0.00 | |
| isorhamnetin-3-O-galactoside | 0.74 ± 0.00 | |
| isorhamnetin-3-O-glucoside | 0.57 ± 0.00 | |
| Phenolic acids | chlorogenic acid | 20.55 ± 0.12 |
| cryptochlorogenic acid | 1.03 ± 0.00 | |
| caffeoylquinic acid | n.d. | |
| p-coumaroylquinic acid | 0.16 ± 0.03 | |
| caffeoyl-dihydroxyphenyl-lactaoyl-tartaric acid | n.d. | |
| 2-O-p-coumaroylglicerol | n.d. | |
| 1-O-p-coumaroylglicerol | n.d. | |
| p-coumaroylspermidin | n.d. | |
| di-p-coumaroylspermidin | n.d. | |
| ferullyquinic acid | n.d. | |
| Flavon-3-ols | (+) catechin | 1.44 ± 0.02 |
| procyanidin B2 | 2.61 ± 0.00 | |
| (−) epicatechin | 0.76 ± 0.00 | |
| Dihydrochalcones | phloretin-2-O-xylosyl-glucoside | 1.48 ± 0.14 |
| phloretin 2-O-glucoside (phloridzin) | 15.52 ± 0.00 |
| Sample | Total Phenolic Content (mg Gallic Acid/100 g d.m.) | Change to Control | Total Flavonoids Content (mg Rutin/100 g d.m.) | TEAC (mg Tx/g d.m.) | Change to Control |
|---|---|---|---|---|---|
| Control | 1.02 ± 0.00 a * | - | n.d. | 0.03 ± 0.00 a * | - |
| GFB5AP | 3.58 ± 0.00 b | 250% | 8.04 ± 0.10 b | 1.97 ± 0.19 b | 6467% |
| GFB10AP | 7.15 ± 1.57 c | 600% | 15.87 ± 0.27 c | 2.26 ± 0.05 c | 7433% |
| GFB15AP | 21.96 ± 2.00 d | 2050% | 21.56 ± 0.31 d | 3.21 ± 0.10 d | 10600% |
| Compounds | Control | GFB5AP | GFB10AP | GFB15AP | |
|---|---|---|---|---|---|
| Flavonols | luteolin 6-C-hexoside O-hexoside | 0.84 ± 0.20 a * | 0.99 ± 0.07 a | 0.97 ± 0.00 a | 1.07 ± 0.03 b |
| luteolin O-hexoside C-hexoside | 0.96 ± 0.00 c | 1.00 ± 0.08 c | 0.88 ± 0.00 b | 0.82 ± 0.02 a | |
| quercetin-O-rutinoside | n.d. | 0.22 ± 0.03 a | 0.44 ± 0.01 b | 0.52 ± 0.05 c | |
| quercetin-3-O-galactoside | 0.11 ± 0.02 a | 1.21 ± 0.17 b | 2.60 ± 0.09 c | 4.37 ± 0.00 d | |
| quercetin-3-O-glucoside | 0.01 ± 0.00 a | 0.25 ± 0.00 b | 0.63 ± 0.00 c | 1.02 ± 0.07 d | |
| quercetin-3-O-arabinoside | 0.08 ± 0.00 a | 0.47 ± 0.03 b | 0.93 ± 0.02 c | 1.72 ± 0.05 d | |
| quercetin-3-O-xyloside | 0.10 ± 0.01 a | 0.83 ± 0.03 b | 1.49 ± 0.00 c | 2.89 ± 0.07 d | |
| quercetin-3-O-rhamnoside | 0.13 ± 0.02 a | 1.06 ± 0.07 b | 2.00 ± 0.00 c | 3.71 ± 0.00 d | |
| isorhamnetin-3-O-galactoside | n.d. | n.d. | 0.10 ± 0.03 a | 0.21 ± 0.00 b | |
| isorhamnetin-3-O-glucoside | n.d. | n.d. | 0.14 ± 0.01 a | 0.15 ± 0.00 a | |
| Phenolic acids | chlorogenic acid | 0.35 ± 0.00 a | 1.33 ± 0.09 b | 2.36 ± 0.00 c | 3.74 ± 0.12 d |
| cryptochlorogenic acid | n.d. | 0.06 ± 0.00 a | 0.12 ± 0.00 b | 0.19 ± 0.04 c | |
| caffeoylquinic acid | 0.42 ± 0.00 a | 0.49 ± 0.03 b | 0.37 ± 0.06 a | 0.57 ± 0.00 c | |
| p-coumaroylquinic acid | 0.07 ± 0.00 a | 0.13 ± 0.00 b | 0.21 ± 0.02 c | 0.32 ± 0.01 d | |
| caffeoyl-dihydroxyphenyl-lactaoyl-tartaric acid | 0.15 ± 0.00 a | 0.28 ± 0.00 b | 0.41 ± 0.00 c | 0.61 ± 0.00 d | |
| 2-O-p-coumaroylglicerol | 0.28 ± 0.00 ab | 0.26 ± 0.02 ab | 0.25 ± 0.00 ab | 0.23 ± 0.01 a | |
| 1-O-p-coumaroylglicerol | 1.39 ± 0.00 a | 1.74 ± 0.06 c | 1.58 ± 0.00 b | 1.54 ± 0.01 b | |
| p-coumaroylspermidin | 0.27 ± 0.05 d | 0.16 ± 0.01 c | 0.11 ± 0.00 b | 0.05 ± 0.00 b | |
| di-p-coumaroylspermidin | 0.98 ± 0.03 a | 1.21 ± 0.12 b | 1.10 ± 0.01 b | 1.12 ± 0.03 b | |
| ferullyquinic acid | 0.16 ± 0.00 a | 0.31 ± 0.01 b | 0.35 ± 0.01 b | 0.34 ± 0.02 b | |
| Flavon-3-ols | (+) catechin | 0.09 ± 0.00 a | n.d. | 0.15 ± 0.01 b | n.d. |
| procyanidin B2 | 0.20 ± 0.00 a | 0.32 ± 0.00 c | 0.28 ± 0.00 b | 0.46 ± 0.00 d | |
| (−) epicatechin | n.d. | n.d. | n.d. | n.d. | |
| Dihydrochalcones | phloretin-2-O-xylosyl-glucoside | 0.02 ± 0.00 a | 0.09 ± 0.00 b | 0.16 ± 0.00 c | 0.25 ± 0.01 d |
| phloretin 2-O-glucoside (phloridzin) | 0.04 ± 0.00 a | 0.84 ± 0.00 b | 1.78 ± 0.03 c | 2.99 ± 0.02 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050807
Gumul D, Ziobro R, Korus J, Kruczek M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants. 2021; 10(5):807. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050807
Chicago/Turabian StyleGumul, Dorota, Rafał Ziobro, Jarosław Korus, and Marek Kruczek. 2021. "Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads" Antioxidants 10, no. 5: 807. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050807