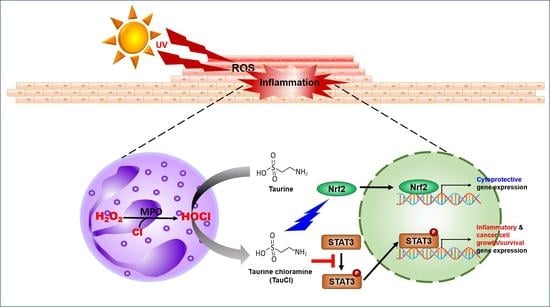
Topically Applied Taurine Chloramine Protects against UVB-Induced Oxidative Stress and Inflammation in Mouse Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. UVB Irradiation and Treatment
2.4. Histology
2.5. Immunohistochemical Analysis
2.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
2.7. Immunofluorescence Staining
2.8. Tissue Lysis and Protein Extraction
2.9. Preparation of Cytosolic and Nuclear Extracts
2.10. Western Blot Analysis
2.11. Real-Time Quantitative PCR (qPCR)
2.12. Statistical Analysis
3. Results
3.1. UVB-Induced Oxidative Stress and Apoptosis Were Attenuated in TauCl-Treated Mice
3.2. UVB-Induced Acute Skin Inflammation Was Ameliorated in TauCl-Treated Mice Skin
3.3. UVB-Induced Phosphorylation of STAT3 Was Blunted by Topical Application of TauCl
3.4. Topical Application of TauCl Upregulates Cytoprotective Gene Expression through Nrf2 Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quaresma, J.A.S. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Handfield, C.; Kwock, J.; MacLeod, A.S. Innate antiviral immunity in the skin. Trends Immunol. 2018, 39, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Ryser, S.; Schuppli, M.; Gauthier, B.; Hernandez, D.R.; Roye, O.; Hohl, D.; German, B.; Holzwarth, J.A.; Moodycliffe, A.M. UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d. J. Investig. Dermatol. 2014, 134, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Andrew, R.; Blaustein, C.S. Ultraviolet radiation. In Encyclopedia of Biodiversity, 2nd ed.; Simon, A.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 296–303. [Google Scholar]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [Green Version]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150434. [Google Scholar] [CrossRef] [Green Version]
- Stengel, F. Homeostasis in topical photoprotection: Getting the spectral balance right. Am. J. Clin. Dermatol. 2018, 19, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Arthur, P.; Terrill, J.; Grounds, M. Taurine: An anti-inflammatory and antioxidant with strong potential benefits for Duchenne muscular dystrophy. Neuromusc. Disord. 2017, 27, S191–S192. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geggel, H.S.; Ament, M.E.; Heckenlively, J.R.; Martin, D.A.; Kopple, J.D. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N. Engl. J. Med. 1985, 312, 142–146. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Cha, Y.N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kang, I.S. Taurine chloramine, a taurine metabolite from activated neutrophils, inhibits osteoclastogenesis by suppressing NFATc1 expression. Adv. Exp. Med. Biol. 2015, 803, 99–107. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. Chemical properties of N-chlorotaurine sodium, a key compound in the human defence system. Arch. Pharm. 2002, 335, 411–421. [Google Scholar] [CrossRef]
- Thomas-Ahner, J.M.; Wulff, B.C.; Tober, K.L.; Kusewitt, D.F.; Riggenbach, J.A.; Oberyszyn, T.M. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007, 67, 3468–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgren, C.A.K.; Sjostrand, D.; Biner, O.; Bennett, M.; Rudling, A.; Johansson, A.L.; Brzezinski, P.; Carlsson, J.; von Ballmoos, C.; Hogbom, M. Scavenging of superoxide by a membrane-bound superoxide oxidase. Nat. Chem. Biol. 2018, 14, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem. Pharmacol. 2003, 66, 1527–1535. [Google Scholar] [CrossRef]
- Gu, M.; Singh, R.P.; Dhanalakshmi, S.; Agarwal, C.; Agarwal, R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007, 67, 3483–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, S.; Alam, S.; Pal, A.; Kumar, M.; Singh, D.; Ansari, K.M. UVB exposure enhanced benzanthrone-induced inflammatory responses in SKH-1 mouse skin by activating the expression of COX-2 and iNOS through MAP kinases/NF-kappaB/AP-1 signalling pathways. Food Chem. Toxicol. 2016, 96, 183–190. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef] [PubMed]
- Froger, N.; Moutsimilli, L.; Cadetti, L.; Jammoul, F.; Wang, Q.P.; Fan, Y.; Gaucher, D.; Rosolen, S.G.; Neveux, N.; Cynober, L.; et al. Taurine: The comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin. Eye Res. 2014, 41, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Guta, R.C. Is taurine a pharmaconutrient? J. Pharmacol. Ther. Res. 2018, 2, 18–20. [Google Scholar] [CrossRef]
- Janeke, G.; Siefken, W.; Carstensen, S.; Springmann, G.; Bleck, O.; Steinhart, H.; Hoger, P.; Wittern, K.P.; Wenck, H.; Stab, F.; et al. Role of taurine accumulation in keratinocyte hydration. J. Investig. Dermatol. 2003, 121, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, P.P.; Molloy, A.M.; Rogers, S.; Bloomfield, F.J. Neutrophil taurine in psoriasis. Ir. J. Med. Sci. 1996, 165, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Degim, Z.; Celebi, N.; Sayan, H.; Babul, A.; Erdogan, D.; Take, G. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids 2002, 22, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Investig. Dermatol. 1993, 100, 35S–41S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gruijl, F.R.; Forbes, P.D. UV-induced skin cancer in a hairless mouse model. Bioessays 1995, 17, 651–660. [Google Scholar] [CrossRef]
- Yum, H.W.; Kim, S.H.; Kang, J.X.; Surh, Y.J. Amelioration of UVB-induced oxidative stress and inflammation in fat-1 transgenic mouse skin. Biochem. Biophys. Res. Commun. 2018, 502, 1–8. [Google Scholar] [CrossRef]
- Kim, D.J.; Angel, J.M.; Sano, S.; DiGiovanni, J. Constitutive activation and targeted disruption of signal transducer and activator of transcription 3 (Stat3) in mouse epidermis reveal its critical role in UVB-induced skin carcinogenesis. Oncogene 2009, 28, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Maiocchi, S.L.; Ku, J.; Thai, T.; Chan, E.; Rees, M.D.; Thomas, S.R. Myeloperoxidase: A versatile mediator of endothelial dysfunction and therapeutic target during cardiovascular disease. Pharmacol. Ther. 2021, 221, 107711. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Cho, I.S.; Park, S.Y.; Schuller-Levis, G.; Levis, W.; Park, E. Taurine chloramine inhibits NO and TNF-alpha production in zymosan plus interferon-gamma activated RAW 264.7 cells. J. Drugs Dermatol. 2011, 10, 659–665. [Google Scholar]
- Walczewska, M.; Marcinkiewicz, J. Taurine chloramine and its potential therapeutical application. Przegl. Lek. 2011, 68, 334–338. [Google Scholar]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.S.; Kim, C. Taurine chloramine administered in vivo increases NRF2-regulated antioxidant enzyme expression in murine peritoneal macrophages. Adv. Exp. Med. Biol. 2013, 775, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, H.U.; Lee, H.N.; Kim, S.H.; Kim, C.; Cha, Y.N.; Joe, Y.; Chung, H.T.; Jang, J.; Kim, K.; et al. Taurine chloramine stimulates efferocytosis through upregulation of Nrf2-mediated heme oxygenase-1 expression in murine macrophages: Possible involvement of carbon monoxide. Antioxid. Redox Signal. 2015, 23, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Kim, S.H.; Jang, J.H.; Kim, C.; Kim, K.; Suh, Y.G.; Joe, Y.; Chung, H.T.; Cha, Y.N.; Surh, Y.J. Role of heme oxygenase-1 in potentiation of phagocytic activity of macrophages by taurine chloramine: Implications for the resolution of zymosan A-induced murine peritonitis. Cell. Immunol. 2018, 327, 36–46. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Yum, H.-W.; Kim, S.H.; Kim, S.-J.; Kim, K.; Kim, C.; Suh, Y.-G.; Surh, Y.-J. Topically Applied Taurine Chloramine Protects against UVB-Induced Oxidative Stress and Inflammation in Mouse Skin. Antioxidants 2021, 10, 867. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060867
Kim SH, Yum H-W, Kim SH, Kim S-J, Kim K, Kim C, Suh Y-G, Surh Y-J. Topically Applied Taurine Chloramine Protects against UVB-Induced Oxidative Stress and Inflammation in Mouse Skin. Antioxidants. 2021; 10(6):867. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060867
Chicago/Turabian StyleKim, Seong Hoon, Hye-Won Yum, Seung Hyeon Kim, Su-Jung Kim, Kyeojin Kim, Chaekyun Kim, Young-Ger Suh, and Young-Joon Surh. 2021. "Topically Applied Taurine Chloramine Protects against UVB-Induced Oxidative Stress and Inflammation in Mouse Skin" Antioxidants 10, no. 6: 867. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060867