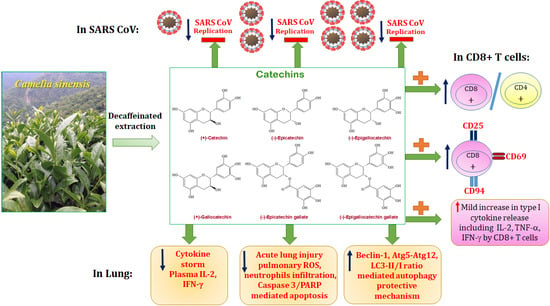
Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Catechins Effects on SARS-CoV Replication In Vero E6 Cells
2.3. Animal Studies and Grouping
2.4. T-Cell Activation
2.5. Immunization and Challenge
2.6. Real-Time Quantitative PCR
2.7. Cytokine ELISA
2.8. Cytokine Array
2.9. Catechins Effects on LPS Induced Cytokine Storm and ALI
2.10. Bronchoalveolar Lavage Fluid (BALF) and Reactive Oxygen Species (ROS) Analysis
2.11. Histopathology of Mouse Lungs
2.12. Immunoblotting of Lung Tissues
2.13. Statistical Analysis
3. Results
3.1. Catechins Decreased CD4+ T Lymphocytes and Increased CD8+ T Lymphocytes Number
3.2. Type I Cytokines in mRNA and Protein Levels Were Enhanced in Catechins-Treated Mice
3.3. Catechins Reduced LPS-Induced ALI and Cytokine Storm
3.4. Catechins Enhanced Autophagy through the PI3K/AKT/mTOR Pathway in LPS-ALI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| BALF | bronchoalveolar lavage |
| C | catechin |
| CAF | caffeine |
| CG | catechin gallate |
| Con A | concanavalin A |
| COVID-19 | coronavirus disease 2019 |
| CPE | cytopathic effects |
| EC | epicatechin |
| ECG | epicatechin gallate |
| EGC | epigallocatechin |
| EGCG | epigallocatechin gallate |
| GC | gallocatechin |
| IFA | immunofluorescence assay |
| LPS | lipopolysaccharide |
| 3-MA | 3-methyladenine |
| MERS | middle east respiratory syndrome |
| MTS | 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| OVA | ovalbumin/alum |
| ROS | reactive oxygen species |
| SARS | severe acute respiratory syndrome |
| SARS-CoV | severe acute respiratory syndrome-associated coronavirus |
References
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; Liu, X.Q.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Masignani, V.; Eickmann, M.; Becker, S.; Abrignani, S.; Klenk, H.D.; Rappuoli, R. SARS—Beginning to understand a new virus. Nat. Rev. Microbiol. 2003, 1, 209–218. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020. [Google Scholar] [CrossRef]
- Rockx, B.; Baas, T.; Zornetzer, G.A.; Haagmans, B.; Sheahan, T.; Frieman, M.; Dyer, M.D.; Teal, T.H.; Proll, S.; van den Brand, J.; et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009, 83, 7062–7074. [Google Scholar] [CrossRef] [Green Version]
- Urra, J.M.; Cabrera, C.M.; Porras, L.; Ródenas, I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. 2020, 217, 108486. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Pooladanda, V.; Thatikonda, S.; Priya Muvvala, S.; Devabattula, G.; Godugu, C. BRD4 targeting nanotherapy prevents lipopolysaccharide induced acute respiratory distress syndrome. Int. J. Pharm. 2021, 601, 120536. [Google Scholar] [CrossRef]
- Qu, L.; Chen, C.; He, W.; Chen, Y.; Li, Y.; Wen, Y.; Zhou, S.; Jiang, Y.; Yang, X.; Zhang, R.; et al. Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. Am. J. Transl Res. 2019, 11, 2042–2055. [Google Scholar]
- Delorme-Axford, E.; Klionsky, D.J. Highlights in the fight against COVID-19: Does autophagy play a role in SARS-CoV-2 infection? Autophagy 2020, 16, 2123–2127. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Karamese, M.; Aydogdu, S.; Karamese, S.A.; Altoparlak, U.; Gundogdu, C. Preventive effects of a major component of green tea, epigallocathechin-3-gallate, on hepatitis-B virus DNA replication. Asian Pac. J. Cancer Prev. 2015, 16, 4199–4202. [Google Scholar] [CrossRef] [Green Version]
- Jena, A.B.; Kanungo, N.; Nayak, V.; Chainy, G.B.N.; Dandapat, J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: Insights from computational studies. Sci. Rep. 2021, 11, 2043. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Park, Y.I.; Cha, Y.E.; Yamamoto, A.; Lee, J.I.; Park, J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021, 547, 23–28. [Google Scholar] [CrossRef]
- Furushima, D.; Ide, K.; Yamada, H. Effect of tea catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules 2018, 23, 1795. [Google Scholar] [CrossRef] [Green Version]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef]
- Hsu, S.P.; Wu, M.S.; Yang, C.C.; Huang, K.C.; Liou, S.Y.; Hsu, S.M.; Chien, C.T. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 2007, 86, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Yano, S.; Ghosh, P.; Kusaba, H.; Buchholz, M.; Longo, D.L. Effect of promoter methylation on the regulation of IFN-gamma gene during in vitro differentiation of human peripheral blood T cells into a Th2 population J. Immunol. 2003, 171, 2510–2516. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Hayakawa, S.; Shimizu, K.; Chien, C.T.; Lai, M.K. Catechins prevents substance P-induced hyperactive bladder in rats via the downregulation of ICAM and ROS. Neurosci. Lett. 2004, 367, 213–217. [Google Scholar] [CrossRef]
- Lin, B.R.; Yu, C.J.; Chen, W.C.; Lee, H.S.; Chang, H.M.; Lee, Y.C.; Chien, C.T.; Chen, C.F. Green tea extract supplement reduces D-galactosamine-induced acute liver injury by inhibition of apoptotic and proinflammatory signaling. J. Biomed. Sci. 2009, 16, 35. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Shun, C.T.; Chien, C.T.; Wang, T.H. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J. Nutr. 2008, 138, 2084–2090. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Chien, C.T. A new approach for the prevention and treatment of Helicobacter pylori infection via upregulation of autophagy and downregulation of apoptosis. Autophagy 2009, 5, 413–414. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Lin, C.Y.; Yang, J.H.; Liou, S.Y.; Li, P.C.; Chien, C.T. Supplementary catechins attenuate cooking-oil-fumes-induced oxidative stress in rat lung. Chin. J. Physiol. 2009, 52, 151–159. [Google Scholar] [CrossRef]
- Leung, W.K.; To, K.F.; Chan, P.K.; Chan, H.L.; Wu, A.K.; Lee, N.; Yuen, K.Y.; Sung, J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 2003, 125, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Faiq, M.A.; Pareek, V.; Raza, K.; Narayan, R.K.; Prasoon, P.; Kumar, P.; Kulandhasamy, M.; Kumari, C.; Kant, K.; et al. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med. Hypotheses 2020, 144, 110271. [Google Scholar] [CrossRef]
- World Health Organization. WHO Environmental Health Team Reports on Amoy Gardens. Available online: http://www.info.gov.hk/gia/general/200305/16/0516114.htm (accessed on 16 May 2003).
- Yamamoto, N.; Yang, R.; Yoshinaka, Y.; Amari, S.; Nakano, T.; Cinatl, J.; Rabenau, H.; Doerr, H.W.; Hunsmann, G.; Otaka, A.; et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004, 318, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Treatment of SARS with human interferons. Lancet 2003, 362, 293–294. [Google Scholar] [CrossRef]
- Wu, C.J.; Jan, J.T.; Chen, C.M.; Hsieh, H.P.; Hwang, D.R.; Liu, H.W.; Liu, C.Y.; Huang, H.W.; Chen, S.C.; Hong, C.F.; et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004, 48, 2693–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Detsika, M.G.; Ampelakiotou, K.; Grigoriou, E.; Psarra, K.; Jahaj, E.; Roussos, C.; Dimopoulou, I.; Orfanos, S.E.; Tsirogianni, A.; Kotanidou, A. A novel ratio of CD8(+):B-cells as a prognostic marker of coronavirus disease 2019 patient progression and outcome. Virology 2021, 556, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [Green Version]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- Sheu, B.C.; Lin, R.H.; Lien, H.C.; Ho, H.N.; Hsu, S.M.; Huang, S.C. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J. Immunol. 2001, 167, 2972–2978. [Google Scholar] [CrossRef] [Green Version]
- Biron, C.A. Expansion, maintenance, and memory in NK and T cells during viral infections: Responding to pressures for defense and regulation. PLoS Pathog. 2010, 6, e1000816. [Google Scholar] [CrossRef]
- Moser, J.M.; Gibbs, J.; Jensen, P.E.; Lukacher, A.E. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat. Immunol. 2002, 3, 189–195. [Google Scholar] [CrossRef]
- Borrego, F.; Robertson, M.J.; Ritz, J.; Peña, J.; Solana, R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology 1999, 97, 159–165. [Google Scholar] [CrossRef]
- Gunturi, A.; Berg, B.E.; Forman, J. Preferential survival of CD8 T and NK cells expressing high levels of CD94. J. Immunol. 2003, 170, 1737–1745. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.W.; Chen, Y.H.; Chuang, Y.C.; Liu, T.-Y.; Hsu, S.-M. CD94 transcripts imply a better prognosis in nasal-type extranodal NK/T-cell lymphoma. Blood 2003, 102, 2623–2631. [Google Scholar] [CrossRef] [Green Version]
- Anfossi, N.; Pascal, V.; Vivier, E.; Ugolini, S. Biology of T memory type 1 cells. Immunol. Rev. 2001, 181, 269–278. [Google Scholar] [CrossRef]
- Braud, V.M.; Aldemir, H.; Breart, B.; Ferlin, W.G. Expression of CD94-NKG2A inhibitory receptor is restricted to a subset of CD8+ T cells. Trends Immunol. 2003, 24, 162–164. [Google Scholar] [CrossRef]
- Tu, P.; Tian, R.; Lu, Y.; Zhang, Y.; Zhu, H.; Ling, L.; Li, H.; Chen, D. Beneficial effect of Indigo Naturalis on acute lung injury induced by influenza A virus. Chin. Med. 2020, 15, 128. [Google Scholar] [CrossRef]
- Mansour, E.; Bueno, F.F.; de Lima-Júnior, J.C.; Palma, A.; Monfort-Pires, M.; Bombassaro, B.; Araujo, E.P.; Bernardes, A.F.; Ulaf, R.G.; Nunes, T.A.; et al. Evaluation of the efficacy and safety of icatibant and C1 esterase/kallikrein inhibitor in severe COVID-19: Study protocol for a three-armed randomized controlled trial. Trials 2021, 22, 71. [Google Scholar] [CrossRef]
- Sohn, K.M.; Lee, S.G.; Kim, H.J.; Cheon, S.; Jeong, H.; Lee, J.; Kim, I.S.; Silwal, P.; Kim, Y.J.; Paik, S.; et al. COVID-19 Patients Upregulate Toll-like Receptor 4-mediated Inflammatory Signaling That Mimics Bacterial Sepsis. J. Korean Med. Sci. 2020, 35, e343. [Google Scholar] [CrossRef]
- Brandão, S.C.S.; Ramos, J.O.X.; Dompieri, L.T.; Godoi, E.T.A.M.; Figueiredo, J.L.; Sarinho, E.S.C.; Chelvanambi, S.; Aikawa, M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2021, 58, 102–110. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Aublin-Gex, A.; Sestito, S.E.; Shirey, K.A.; Patel, M.C.; André, P.; Blanco, J.; Vogel, S.N.; Peri, F.; Lotteau, V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017, 7, 40791. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediators Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef]
- Song, J.; Zhang, L.; Xu, Y.; Yang, D.; Yang, S.; Zhang, W.; Wang, J.; Tian, S.; Yang, S.; Yuan, T.; et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021, 183, 114302. [Google Scholar] [CrossRef]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Munz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024.47. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J. Allergy Clin. Immunol. 2004, 113, 1211–1217. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Wu, C.-J.; Chien, C.-Y.; Chien, C.-T. Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice. Antioxidants 2021, 10, 928. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060928
Yang C-C, Wu C-J, Chien C-Y, Chien C-T. Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice. Antioxidants. 2021; 10(6):928. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060928
Chicago/Turabian StyleYang, Chih-Ching, Chang-Jer Wu, Chen-Yen Chien, and Chiang-Ting Chien. 2021. "Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice" Antioxidants 10, no. 6: 928. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060928