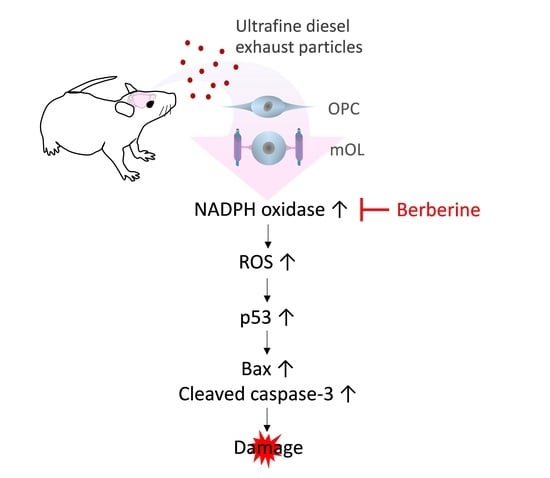
Ultrafine Diesel Exhaust Particles Induce Apoptosis of Oligodendrocytes by Increasing Intracellular Reactive Oxygen Species through NADPH Oxidase Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing of Rodent Brain Cells
2.2. Identification of Brain Cell Types
2.3. Preparation and Exposure to a Working Suspension of Ultrafine DEPs and NOX2 Inhibition
2.4. Measurement of ROS Generation
2.5. Measurement of Cell Viability
2.6. Measurement of Apoptosis
2.7. Hoechst Staining to Identify Dead Cells
2.8. Animals and Exposure to Ultrafine DEPs
2.9. Preparation of Tissue
2.10. Immunofluorescence Staining
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. Identification of Brain Cell Types
3.2. ROS Generation According to Concentration of Ultrafine DEPs
3.3. Gp91phox Expression after Exposure to Ultrafine DEPs
3.4. ROS Generation by NOX2 after Exposure to Ultrafine DEPs
3.5. Expression of p53, Bax, Bcl-2, and Cleaved Caspase-3 after Exposure to Ultrafine DEPs
3.6. Decrease in Cell Viability after Exposure to Ultrafine DEPs
3.7. Damage of gp91phox-Positive Oligodendrocytes in Cerebellar White Matter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, E.M.; Breznan, D.; Karthikeyan, S.; Mackinnon-Roy, C.; Charland, J.-P.; Dabek-Zlotorzynska, E.; Celo, V.; Kumarathasan, P.; Brook, J.R.; Vincent, R. Cytotoxic and inflammatory potential of size-fractionated particulate matter collected repeatedly within a small urban area. Part. Fibre Toxicol. 2015, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemmar, A.; Hoet, P.M.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Sun, W.; Li, F.; Shi, M.; Meng, X.; Wang, C.; Meng, M.; Tang, W.; Liu, H.; Wang, L.; et al. The harmful effects of acute PM2.5 exposure to the heart and a novel preventive and therapeutic function of CEOs. Sci. Rep. 2019, 9, 3495. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.-C.; Dao, K.; Roqué, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Hamanaka, R.B.; Mutlu, G.M. Particulate matter air pollution: Effects on the cardiovascular system. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69. [Google Scholar] [CrossRef]
- Bhatt, D.P.; Puig, K.L.; Gorr, M.W.; Wold, L.E.; Combs, C.K. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS ONE 2015, 10, e0127102. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Myung, W.; Kim, D.K.; Kim, S.E.; Kim, C.T.; Kim, H. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci. Rep. 2017, 7, 44741. [Google Scholar] [CrossRef] [Green Version]
- Tateo, F.; Grassivaro, F.; Ermani, M.; Puthenparampil, M.; Gallo, P. PM2.5 levels strongly associate with multiple sclerosis prevalence in the Province of Padua, Veneto Region, North-East Italy. Mult. Scler. 2019, 25, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.; Geng, X.; Li, F.; Ding, Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front. Cell. Neurosci. 2017, 10, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef]
- Hartz, A.M.; Bauer, B.; Block, M.L.; Hong, J.S.; Miller, D.S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008, 22, 2723–2733. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; White, E. p53-dependent apoptosis pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Surace, M.J.; Block, M.L. Targeting microglia-mediated neurotoxicity: The potential of NOX2 inhibitors. Cell. Mol. Life Sci. 2012, 69, 2409–2427. [Google Scholar] [CrossRef] [Green Version]
- Thorburne, S.K.; Juurlink, B.H. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J. Neurochem. 1996, 67, 1014–1022. [Google Scholar] [CrossRef]
- Back, S.A.; Gan, X.; Li, Y.; Rosenberg, P.A.; Volpe, J.J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998, 18, 6241–6253. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.Y.; Sohn, H.J.; Kim, S.W.; Kim, C.H.; Ahn, H.Y.; Kim, D.W.; Cho, S.S.; Seo, J.H. Differential expression of alpha B-crystallin causes maturation-dependent susceptibility of oligodendrocytes to oxidative stress. BMB Rep. 2013, 46, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kim, C.H.; Lee, E.Y.; Seo, J.H. Alpha B-crystallin overexpression protects oligodendrocyte precursor cells against oxidative stress-induced apoptosis through the Akt pathway. J. Mol. Neurosci. 2020, 70, 751–758. [Google Scholar] [CrossRef]
- Ruuls, S.R.; Bauer, J.; Sontrop, K.; Huitinga, I.; A’t Hart, B.; Dijkstra, C.D. Reactive oxygen species are involved in the pathogenesis of experimental allergic encephalomyelitis in Lewis rats. J. Neuroimmunol. 1995, 56, 207–217. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.-H.; Kim, Y.-D.; Seo, J.H. High vulnerability of oligodendrocytes to oxidative stress induced by ultrafine urban particles. Antioxidants 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004, 18, 1618–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Zhou, B.; Qiu, Y.; Wu, N.; Chen, A.D.; Zhou, H.; Chen, Q.; Kang, Y.M.; Li, Y.H.; Zhu, G.Q. FNDC5 attenuates oxidative stress and NLRP3 inflammasome activation in vascular smooth muscle cells via activating the AMPK-SIRT1 signal pathway. Oxid. Med. Cell. Longev. 2020, 2020, 6384803. [Google Scholar] [CrossRef]
- Haan, C.; Behrmann, I. A cost effective non-commercial ECL-solution for Western blot detections yielding strong signals and low background. J. Immunol. Methods 2007, 318, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Underwood, E. The polluted brain. Science 2017, 355, 342–345. [Google Scholar] [CrossRef]
- Heydarpour, P.; Amini, H.; Khoshkish, S.; Seidkhani, H.; Sahraian, M.A.; Yunesian, M. Potential impact of air pollution on multiple sclerosis in Tehran, Iran. Neuroepidemiology 2014, 43, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-W.; Lee, T.-L.; Chen, Y.-C.; Liang, C.-J.; Wang, S.-H.; Lue, J.-H.; Tsai, J.-S.; Lee, S.-W.; Chen, S.-H.; Yang, Y.-F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Thorburne, S.K.; Hertz, L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 1998, 22, 371–378. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.H.; Nguyen, U.T.; Kim, Y.-H.; Shin, H.-M.; Yang, I.-J. Astragali Radix and its compound formononetin ameliorate diesel particulate matter-induced skin barrier disruption by regulation of keratinocyte proliferation and apoptosis. J. Ethnopharmacol. 2019, 228, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Soberanes, S.; Panduri, V.; Mutlu, G.M.; Ghio, A.; Bundinger, G.R.S.; Kamp, D.W. p53 Mediates Particulate Matter–induced Alveolar Epithelial Cell Mitochondria-regulated Apoptosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1229–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Deng, Z.; Feng, Y.; Liao, F.; Zhou, F.; Feng, S.; Wang, X. PM 2.5 induced apoptosis in endothelial cell through the activation of the p53-bax-caspase pathway. Chemosphere 2017, 177, 135–143. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, J.H.; Kho, A.R.; Kim, I.Y.; Lee, S.H.; Lee, B.E.; Choi, E.; Sohn, M.; Stevenson, M.; Chung, T.N.; et al. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J. Neuroinflamm. 2015, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Baud, O.; Vartanian, T.; Volpe, J.J.; Rosenberg, P.A. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 9936–9941. [Google Scholar] [CrossRef] [Green Version]
- Ai, X.; Yu, P.; Peng, L.; Luo, L.; Liu, J.; Li, S.; Lai, X.; Luan, F.; Meng, X. Berberine: A review of its pharmacokinetics properties and therapeutic potentials in diverse vascular diseases. Front. Pharmacol. 2021, 12, 762654. [Google Scholar] [CrossRef]
- Sarna, L.K.; Wu, N.; Hwang, S.Y.; Siow, Y.L.; Karmin, O. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can. J. Physiol. Pharmacol. 2010, 88, 369–378. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Kim, J.-H.; Kim, Y.-D.; Seo, J.H. Ultrafine Diesel Exhaust Particles Induce Apoptosis of Oligodendrocytes by Increasing Intracellular Reactive Oxygen Species through NADPH Oxidase Activation. Antioxidants 2022, 11, 1031. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11051031
Kim JY, Kim J-H, Kim Y-D, Seo JH. Ultrafine Diesel Exhaust Particles Induce Apoptosis of Oligodendrocytes by Increasing Intracellular Reactive Oxygen Species through NADPH Oxidase Activation. Antioxidants. 2022; 11(5):1031. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11051031
Chicago/Turabian StyleKim, Ji Young, Jin-Hee Kim, Yong-Dae Kim, and Je Hoon Seo. 2022. "Ultrafine Diesel Exhaust Particles Induce Apoptosis of Oligodendrocytes by Increasing Intracellular Reactive Oxygen Species through NADPH Oxidase Activation" Antioxidants 11, no. 5: 1031. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11051031