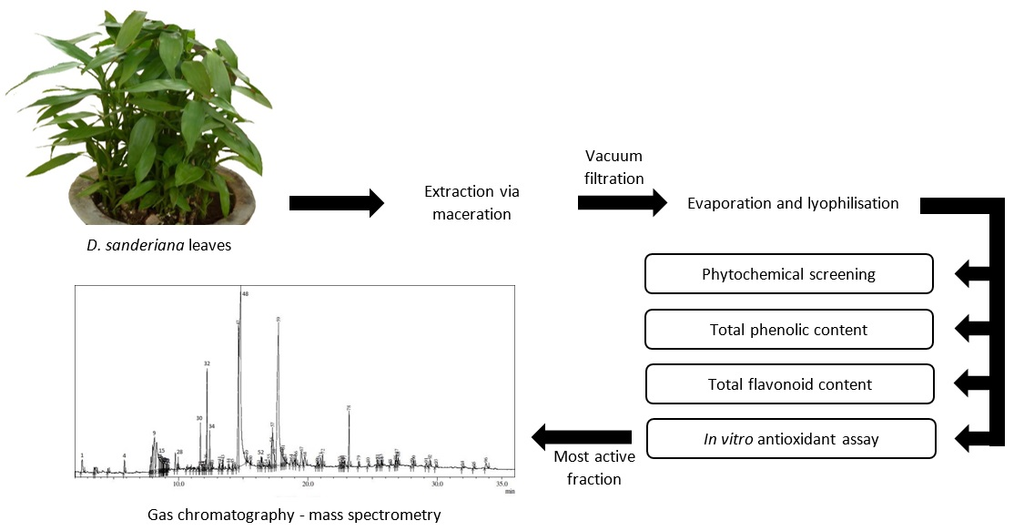
3.1. Extraction Yield and Pharmacognostic Study
A mixture of water and ethanol was used for extraction, as they offer low toxicity and high extraction yield. Moreover, they can be mixed in different hydroethanolic ratios, which enables the modulation of the polarity of the solvent.
Table 1 shows the weight and percentage yield of the E100, E80, E60, E40, E20, and E0 fraction extracts. The E100 had the highest extract yield (4.29 g) and the E20 had the lowest extract yield (0.82 g). Thus, 100% ethanol was the best solvent for extraction.
The pharmacognostic study of
D. sanderiana leaves showed that both powder and extracts contain a large amount of phytonutrients. The classes of phytochemical groups present are shown in
Table 2. All extracts and powder of the plant were positive for tannins, saponins, terpenoids, cardiac glycosides, and quinone. Alkaloids and phlobatannins were absent in the plant.
3.3. TPC
Natural phenolics have strong antioxidant properties since these molecules are able to terminate the generation of free radical chain reactions in the presence of hydroxyl groups which act as reducing agents [
19]. All phenolic samples, including flavonoids, anthocyanins, and non-flavonoid phenolic compounds, were measured by the Folin-Ciocalteu method [
20].
The TPC in the extracts was calculated from the regression equation (
y = 5.0739
x;
R2 = 0.9855) of the calibration curve. The TPC of plant extracts ranged from 36.93 to 151.14 mg GAE/g dE (
Table 4). E60 had the highest TPC.
A previous study showed that phenolic compounds are directly associated with free radical-scavenging activity [
21]. However, the accuracy for the determination of TPC using the Folin-Ciocalteu assay can be affected by various non-phenolic–reducing compounds, for instance organic acid and ascorbic acids, and thus leads to overestimation of the TPC [
22]. Moreover, underestimation of the TPC will occur since various types of phenolics react differently with the Folin-Ciocalteu reagent, particularly several phenolic compounds which exhibit low absorption [
22].
3.4. DPPH
DPPH is a stable nitrogen-centred free radical that acts as an electron acceptor or hydrogen radical. It is reduced to a stable diamagnetic molecule in the presence of an antioxidant molecule and changes colour from purple to yellow. The DPPH assay is a convenient method to evaluate the free radical–scavenging ability of various potent antioxidant sources, especially medicinal plants [
23].
In this study the DPPH assay was performed using BHA as the standard. To maintain the stability of free radicals, the DPPH assay was carried out at room temperature [
24].
Table 5 shows the percentage inhibition of
D. sanderiana extracts and BHA. The concentration of various extracts will influence the free radical–scavenging activity [
23]. The increasing concentration of the extracts from 0.05 to 1 mg/mL will gradually increase the percentage of inhibition by the DPPH assay as shown in
Table 5.
Table 6 shows the IC
50 values for the extracts. A lower IC
50 indicates higher antioxidant activities. The E60 extract showed the highest percentage inhibition at 1 mg/mL and the lowest IC
50 [
21].
TFC, TPC, and antioxidant activities of different species of
Dracaena were evaluated previously.
Dracaena cambodiana was found to possess a significant amount of TPC and TFC and exhibited antioxidant activity with an IC
50 of 1.61 mg/mL from ethyl acetate extract [
25].
Dracaena draco fruit extract showed an IC
50 of 0.30 mg/mL, lower than the reference used in the study [
26]. Shukla et al. reported an IC
50 value of 0.46 mg/mL for the
Dracaena reflexa stem dichloromethane extract [
27].
Dracaena umbratica extracts from leaves, rhizomes, and roots showed antioxidant activities with a low percentage of inhibition [
28]. Even though the IC
50 of E60 of
D. sanderiana extract was higher compared to
D. draco fruit extract and
D. reflexa stem extract,
D. sanderiana showed a lower IC
50 value compared to
D. cambodiana.
Previous studies showed that the presence of a hydroxyl group on the antioxidant molecule will contribute to the reduction mechanism of DPPH and the antioxidant activity of the plant extract [
29]. Numerous phytochemicals, such as phenolics, flavonols, carotenoids, and tannins, might contribute to the antioxidant activity of the extracts. In the present study, the extracts from the leaves of
D. Sanderiana exhibited high antioxidant activity. The E60 extract was the most active fraction, with 91.71% inhibition and an IC
50 value of 0.5 mg/mL. Moreover, the E60 extract had a comparatively high TPC (151.14 mg GAE/g dE) and a comparatively low TFC (169.48 mg QE/g dE). The results indicated the major antioxidant components might not be flavonoids but might be those other bioactive groups, such as tannins, quinones, cardiac glycosides, saponins, terpenoids, and other phenolic compounds. Furthermore, synergistic effects among the bioactive compounds might affect the high level of antioxidant activity [
30]. The presence of glycerol, 2,3-dihydro-3,5-dihydroxy-6-methyl-(4H)-pyran-4-one,
n-dodecanoic acid, tetradecanoid acid, (
n-) hexadecanoid acid,
n-octadecanoic acid, and phenols also might contribute to high antioxidant activity [
31,
32].
The plant extracts were tested at 1 mg/mL, which is a high concentration for the DPPH assay; however, they did not reach the plateau stage for the percentage inhibition of DPPH. In contrast, the percentage inhibition of DPPH of the BHA standard reached the plateau stage at 0.2 mg/mL, and its IC50 was lower than that of the tested extracts. Thus, the results showed that the standard had higher antioxidant activity than the plant extracts. This is because BHA is a pure synthetic antioxidant compound, whereas the plant extract contains mixtures of bioactive compounds. In future studies, the compounds need to be isolated in order to measure the exact antioxidant activity of the plant.
3.5. GC-MS Analysis
Ninety-six constituents in E60, the most active extract, were identified by GC-MS analysis (
Figure 1). The identified compounds with a matching value of 90% and above are reported in
Table 7. Several of the identified compounds associated with antioxidant activity have been reported in previous studies. For example, Jerzykiewicz et al. [
33] reported that glycerol was found to be a strong antioxidant and Yu et al. [
32] showed that 2,3-dihydro-3,5-dihydroxy-6-methyl-(4H)-pyran-4-one has strong antioxidant properties in glucose-histidine Maillard reaction products. Henry et al. [
31] reported a few compounds that have high antioxidant activity. For instance, lauric acid (
n-dodecanoic acid) has 60% antioxidant activity in several food products, such as coconut and soybean products, myristic acid (tetradecanoid acid) has 71% antioxidant activity, and palmitic acid or (
n-) hexadecanoid acid has 68% antioxidant activity. In contrast, oleic acid has only moderate activity, and stearic acid (
n-octadecanoic acid) has poor antioxidant activity.