1. Introduction
Marburg virus (MARV)—like the related Ebola virus (EBOV)—is a negative-sense RNA virus that belongs to the family
Filoviridae [
1]. Like EBOV, MARV causes severe viral hemorrhagic fever in humans, with high case fatality rates. Clinically, Marburg virus disease (MVD) is characterized by influenza-like symptoms and a high fever that progresses rapidly towards gastrointestinal and neurological dysfunction, as well as blood coagulation abnormalities and hemorrhagic manifestations [
2,
3]. This pathology seems to be driven by the damage caused by rampant and systemic virus replication in combination with an excessive inflammatory response. In severe cases, death results from multiple organ dysfunction and shock.
Since the discovery of MARV over 50 years ago, there have been 14 recorded outbreaks of MVD, with an overall case fatality rate of ~81% [
1]. The largest and most severe outbreak occurred in Angola between 2004 and 2005, resulting in 252 cases and 227 deaths [
4]. The Angola variant of MARV (MARV/Ang) is derived from this outbreak and thought to be among the most virulent of all MARV variants [
5]. Notably, a single fatal case of MVD was recently identified in Guinea [
6], making this the first report of MARV in a human in West Africa and underscoring the threat that this virus poses to public health.
The World Health Organization lists MVD as one of a few priority diseases requiring urgent research and development attention [
7]. Yet, despite the continuous threat of MARV outbreaks, there are still no licensed vaccines or approved countermeasures for MVD. Nevertheless, several vaccine candidates have been developed and many have shown promising efficacy in various animal models [
8]. Among the most promising vaccine candidates are those based on the recombinant vesicular stomatitis virus (rVSV) vector platform. In general, two approaches have been taken to generate replicating rVSV-based MARV vaccines that elicit a potent and protective immune response directed against the MARV glycoprotein (GP), the viral protein responsible for virion entry and fusion [
3]. In the first approach, the native VSV glycoprotein (G) gene is replaced with the MARV GP gene to generate live-attenuated vaccines broadly referred to as rVSVΔG-MARV [
9]. An analogous rVSV-based vaccine against EBOV has proven highly efficacious and was recently approved for clinical use by the US FDA [
10]. In the second approach, the MARV GP gene is inserted into a VSV vector with a rearranged gene order that retains expression of a truncated VSV G; this live-attenuated vaccine is referred to as rVSVN4CT1-MARV [
11].
Preclinical work in nonhuman primates (NHPs) has demonstrated that a single dose of rVSVΔG-MARV expressing GP from the MARV variant Musoke (MARV/Mus) confers 100% protection against challenge with the homologous virus [
12], offering durable immunity lasting at least 14 months [
13] and an exceptional safety profile [
14]. Post-exposure efficacy has also been demonstrated using this vaccine, with 100% survival in animals vaccinated 30 min after infection with MARV/Mus [
15], 83% survival in animals vaccinated after 24 h [
16], and 33% survival in animals vaccinated after 48 h [
16]. Although the MARV/Mus-based vaccine is effective in NHPs challenged with MARV/Ang or the related Ravn virus [
17], the efficacy of a MARV/Ang-based vaccine and MARV/Ang challenge remains relatively understudied. To date, one study has demonstrated complete protection in NHPs immunized with an rVSVΔG-MARV vaccine expressing MARV/Ang GP and challenged with MARV/Ang [
18]. A follow-up study in the same NHP model demonstrated the ability of this vaccine to confer rapid protection, with 100% survival following challenge (1000 PFU, IM) as early as 7 days postvaccination and 75% survival at 3 days postvaccination [
19]. These results highlight the potential of this vaccine for rapid intervention in an outbreak setting. Interestingly, a separate study showed limited postexposure efficacy in NHPs, with only 25% of animals surviving when vaccinated 20–30 min after a high-dose challenge of MARV/Ang [
20] in contrast to the results observed for MARV/Mus challenge [
12]. Postexposure protection increased to 75% when a lower dose of MARV/Ang was used for challenge [
20]. Notably, the rVSVN4CT1-MARV vaccine expressing MARV/Ang GP also showed effective postexposure protection against a low dose (50 PFU, IM) of MARV/Ang, with 60% of animals surviving challenge [
20]. The same rVSVN4CT1-MARV vaccine has also been shown to protect NHPs against MARV/Ang (1000 PFU, IM) when administered as part of a trivalent (EBOV, MARV, and Sudan virus) filovirus vaccine [
11]. Advanced development of these MARV vaccine candidates is now progressing, as preparedness to counteract the threat of emerging diseases of epidemic or pandemic potential has become an urgent priority [
21].
In this study, we sought to advance the preclinical development of an rVSVΔG-MARV/Ang vaccine termed PHV01. In particular, we evaluated three vaccine virus materials: (1) an uncloned research virus stock at passage level 3 (rVSVΔG-MARV P3); (2) a cloned (i.e., plaque-purified) GMP-produced Master Viral Seed (PHV01 MVS) at passage level 9; and (3) a GMP-produced cloned Formulated Drug Substance (PHV01 FDS) at passage level 11 (
Figure S1). The vaccine materials were tested in the well-defined guinea pig model, which uses guinea-pig-adapted MARV/Ang (GPA-MARV/Ang) as the challenge virus to produce a uniformly lethal infection that recapitulates several hallmarks of MVD, including lymphocytopenia, thrombocytopenia, high viremia, and systemic spread of virus [
22]. Following immunization with a single 2 × 10
6 PFU dose, all three vaccines completely protected guinea pigs from lethal MARV challenge. A dose level of 2 × 10
4 PFU conferred complete or partial protection, depending on the vaccine material used, and a dose level of 2 × 10
2 PFU conferred significant but partial protection. Not only does this study further demonstrate the protective efficacy of the rVSV-based MARV vaccine, but it also specifically demonstrates the efficacy of the PHV01 FDS vaccine clone, which was produced via a representative clinical GMP manufacturing process. The results presented here are important to the ongoing development of this promising vaccine candidate and a step towards achieving a safe and effective single-dose vaccine capable of preventing MVD.
2. Materials and Methods
2.1. Animal Ethics and Biosafety Statement
Animal experiments were reviewed and approved by the Animal Care Committee of the Canadian Science Centre for Human and Animal Health (CSCHAH), Winnipeg, Manitoba, in accordance with guidelines from the Canadian Council on Animal Care. All staff working on animal experiments completed education and training programs according to the standard protocols appropriate for this level of biosafety. All work with infectious MARV was performed in the containment level (CL)-4 laboratories at the CSCHAH in accordance with standard operating protocols.
2.2. Viruses and Cells
Vero E6 cells were obtained from the American Type Culture Collection and maintained at 37 °C and 5% CO2 in Dulbecco’s modified Eagle medium (DMEM; HyClone) supplemented with 5% heat-inactivated fetal bovine serum (HI-FBS; HyClone), 2 mM L-glutamine (HyClone), 50 U/mL penicillin, and 50 ug/mL streptomycin (HyClone). Guinea-pig-adapted Marburg virus variant Angola (GPA-MARV/Ang; Marburg virus/NML/C.porcellus-lab/AGO/2005/Ang-GA-P2; GenBank accession no. MF939097) was generated as previously described [
22].
2.3. VSV-Based Vaccines
This study evaluated the protective efficacy of a recombinant vesicular stomatitis virus (rVSVΔG)-based, live attenuated vaccine against MARV. This vaccine, referred to as PHV01, expresses the MARV variant Angola glycoprotein (GP) in place of the VSV glycoprotein (G). The virus was generated via reverse genetics rescue essentially as described previously for a similar construct expressing the MARV variant Musoke GP [
12]. A passage level 2 (P2) stock of virus was derived from the rescued virus (considered P0) via amplification on Vero E6 cells at the Public Health Agency of Canada. The P2 stock virus was subsequently amplified and plaque purified in qualified CGMP Vero cells in proprietary serum-free tissue culture media to generate premaster viral seeds at P8. A single P8 clone (Clone 5) was chosen based on plaque morphology, growth kinetics and productivity in Vero cells, and MARV GP transgene sequence fidelity. This clone was amplified to P9 under CGMP conditions to generate the PHV01 MVS. PHV01 MVS release criteria included the well-defined requirements for GMP viral seed manufacturing for purity (including adventitious agents), potency/strength, identity, and sterility. PHV01 MVS was further amplified to P10 under CGMP conditions to generate the PHV01 Working Viral Seed (WVS). The PHV01 Formulated Drug Substance (FDS) at P11 was produced from the PHV01 WVS in a disposable bioreactor using the same CGMP Vero cells and proprietary media described above. PHV01 FDS was harvested from cell culture medium containing virus, clarified, and further purified by nuclease digestion, depth filtration, and tangential flow ultrafiltration/diafiltration in a recombinant human albumin and Tris buffer formulation. Separately, an rVSVΔG-MARV P3 virus was generated from the initial P2 stock virus for research purposes, and PHV01 MVS was passaged three additional times (PHV01 MVS+3) to P12 (
Figure S1).
Next-generation sequencing was used to confirm the sequence identities of the following viruses: the uncloned P2 stock virus, the premaster viral seeds (P8), PHV01 MVS (P9), PHV01 WVS (P10), and PHV01 MVS+3 (P12). In particular, PHV01 MVS and PHV01 WVS demonstrated 100% and 99.99% identity to the full-length genome plasmid used to rescue the virus, and no high-frequency mutations were identified in the MARV GP coding sequence. These data also highlight the exceptional stability of the rVSV genome over multiple passages (
Figure S1).
The following vaccines were evaluated in this study: rVSVΔG-MARV P3, PHV01 MVS (P9), and PHV01 FDS (P11).
2.4. Guinea Pig Study Design
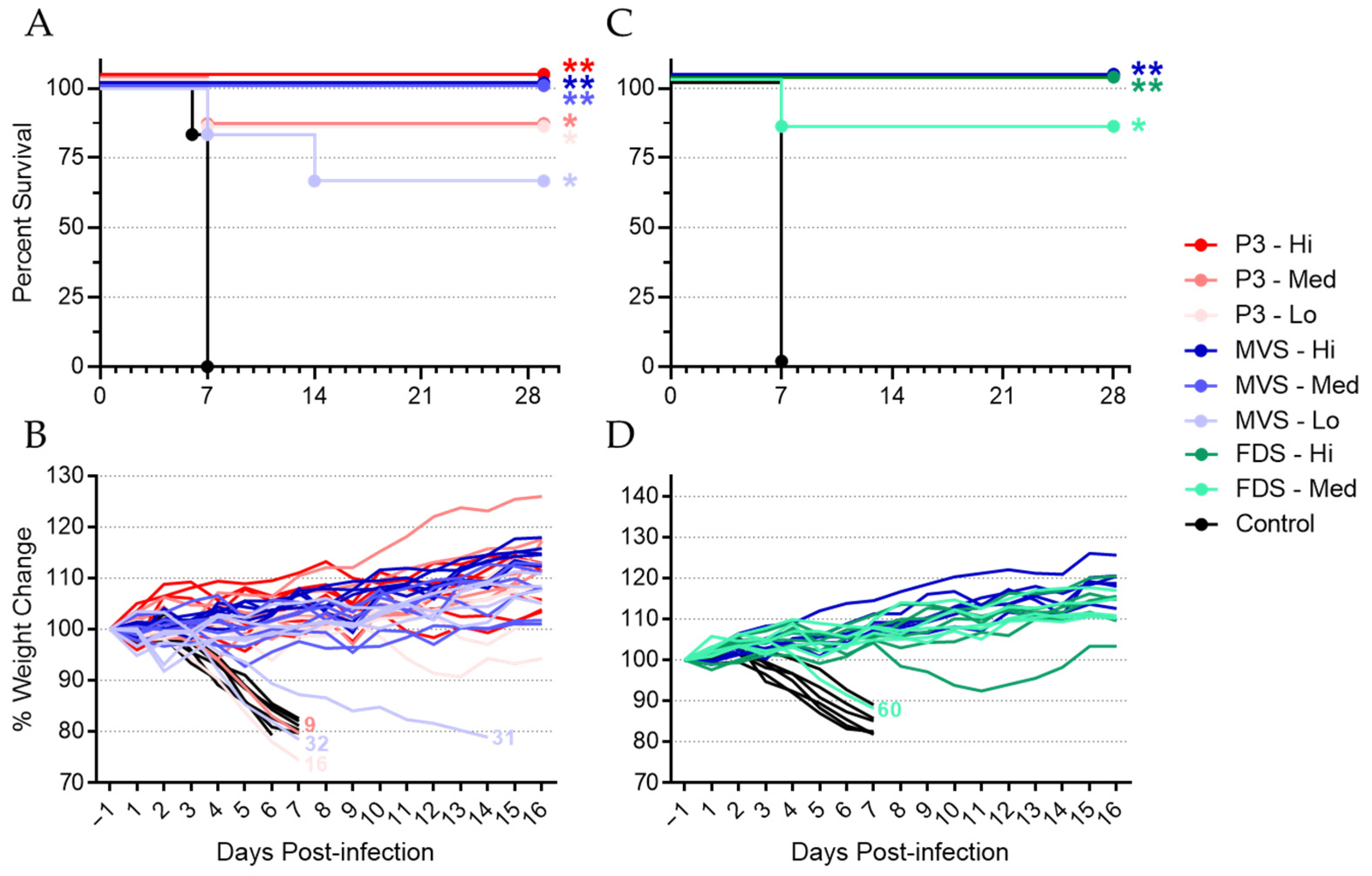
This study was composed of two experiments, termed Experiment #1 and Experiment #2. Both were designed to test the ability of PHV01 to protect female Hartley guinea pigs (Charles River Laboratories) from morbidity and mortality resulting from inoculation with a lethal dose of GPA-MARV/Ang. In Experiment #1, rVSVΔG-MARV research virus P3 and PHV01 MVS were evaluated at three different dose levels: 2 x 106 plaque-forming units (PFU; high), 2 × 104 PFU (medium), and 2 × 102 PFU (low). Groups of 6 guinea pigs were immunized with one of the two vaccine preparations at one of the three specified doses (prepared with sterile, nontoxic, nonpyrogenic 0.9% saline as diluent), while a control group of 6 animals received an equivalent volume of 0.9% saline. In Experiment #2, the PHV01 FDS was evaluated at two different doses, high and medium (as described above), and PHV01 MVS was evaluated again at the high dose for comparative purposes. Groups of 6 guinea pigs were immunized with one of the two vaccine preparations at the specified doses (as described above), and a control group of 6 animals receiving 0.9% saline was also included. All vaccines were administered intramuscularly (IM) in a total volume of 300 µL, with 150 µL delivered to each of the rear quadriceps muscles. Twenty-eight (28) days postvaccination (DPV), animals were inoculated with 1000 times the median lethal dose (LD50) of GPA-MARV/Ang via intraperitoneal (IP) injection. Animals were monitored for disease and survival up to 29 days postinfection (DPI), equivalent to 57 DPV in Experiment #1 and up to 28 DPI (56 DPV) in Experiment #2. EDTA blood and plasma (Experiment #1) or serum (Experiment #2) samples were obtained at 0 DPV (prior to vaccination), 2 DPV, 27 DPV, 5 DPI (33 DPV), and 29 or 28 DPI (57 or 56 DPV).
2.5. VSV and MARV RNA Quantification
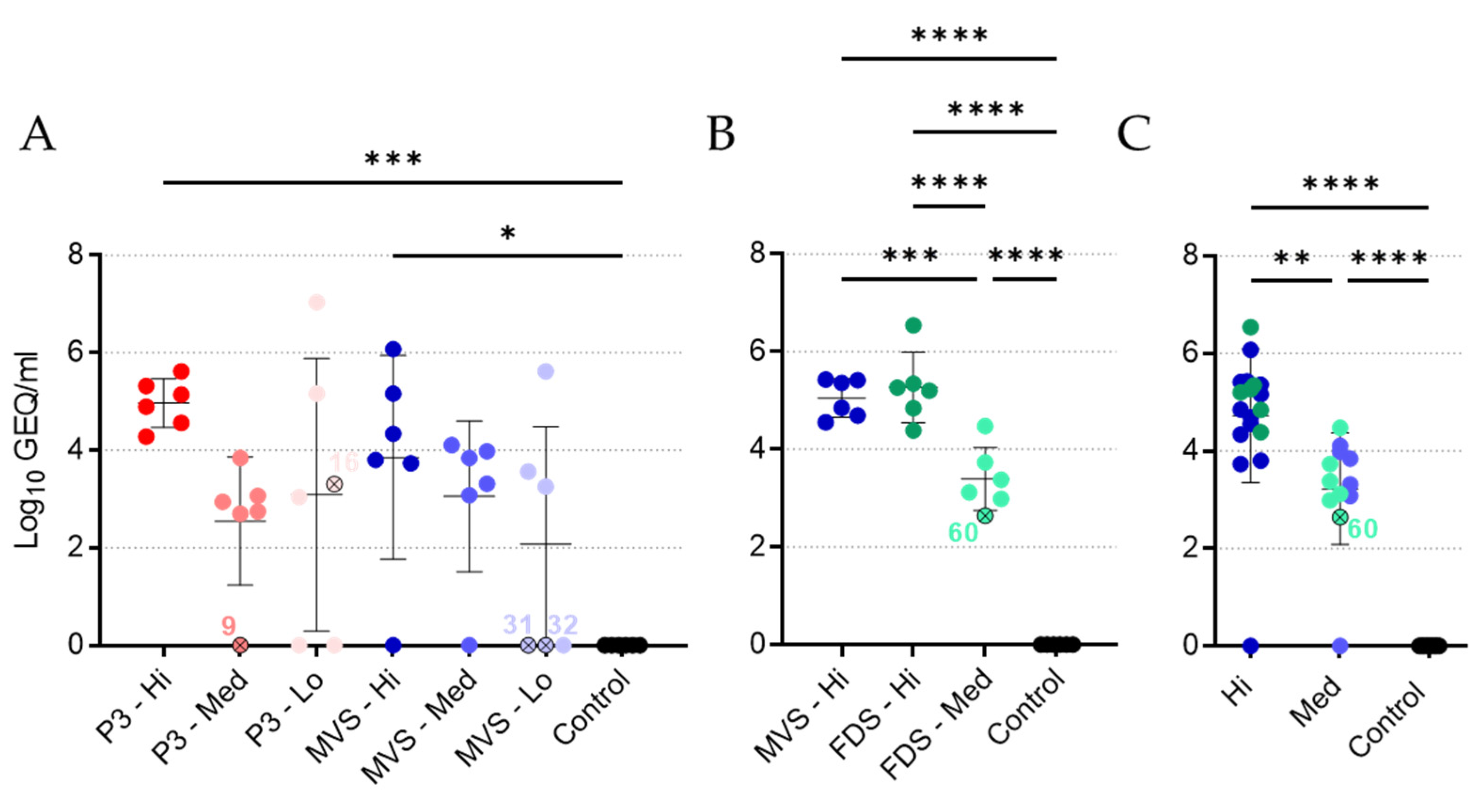
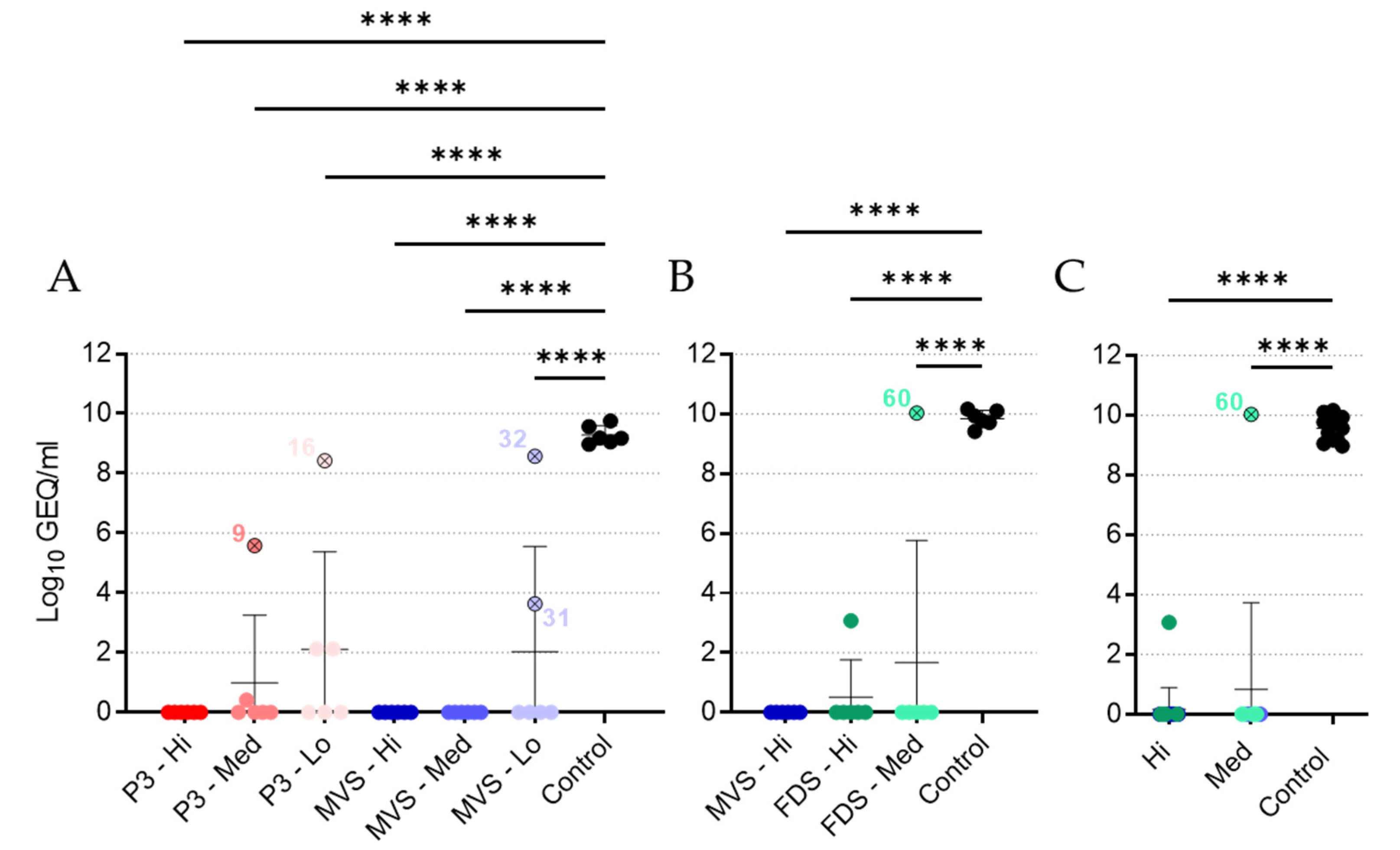
rVSVΔG-MARV (P3, PHV01 MVS, and PHV01 FDS) and MARV RNA were extracted from EDTA blood samples using the QIAamp viral RNA minikit (Qiagen) according to the manufacturer’s instructions (Qiagen). Viral RNA levels were determined by reverse transcription quantitative PCR (RT-qPCR) using the LightCycler 480 thermal cycler (Roche) and the LightCycler 480 RNA Master Hydrolysis Probes kit (Roche), along with the following primers and probe specific for MARV GP: forward primer, 5′-GTRTGCTCCGGRACYCTCCA-3′; reverse primer, 5′-YTGCCCRCTCAGTGTRAATC-3′; probe, 5′-6-FAM-RAARACAGA/ZEN/AGAYGTYCATCTGATGG-IABkFQ-3′. Cycling conditions were as follows: 63 °C for 3 min and 95 °C for 30 s, followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s. RT-qPCR cycle threshold (Ct) values were converted to genome equivalents per milliliter (GEQ/mL) using a standard curve generated from a plasmid containing the MARV genome.
2.6. IgG ELISAs
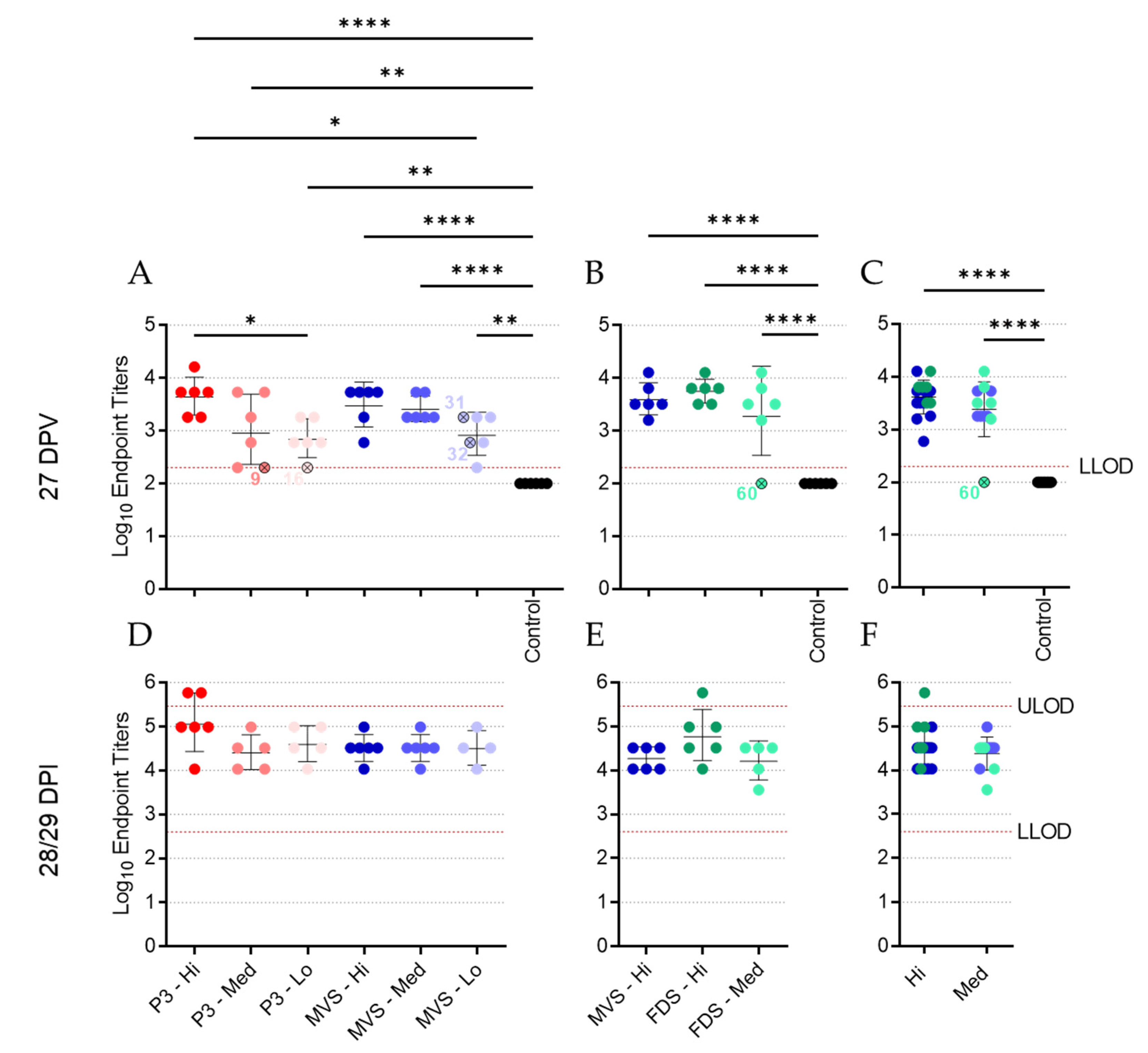
MARV GP-specific IgG was quantified in Lithium–heparin plasma (Experiment #1) or serum (Experiment #2) samples by indirect ELISA. Half-area high-binding 96-well assay plates (Corning) were coated using recombinant MARV GP protein with the transmembrane domain deleted (IBT, Cat. No. 0506-015) prepared in pH 9.5 carbonate–bicarbonate buffer (30 ng/well) at 4 °C overnight. After removing the coating solution, plates were incubated with 5% skim milk (BD Biosciences) prepared in PBS for 1 h at 37 °C. Serial dilutions of plasma or serum samples prepared in 2% skim milk in PBS were then applied to the plates at 4 °C overnight. Following washes with 0.1% Tween-20 in PBS, plates were incubated for 1 h at 37 °C with an HRP-conjugated goat anti-guinea pig IgG (H+L) secondary antibody (KPL, Cat. No. 14-17-06) diluted to 1:1000 in 2% skim milk in PBS. After an additional set of washes with 0.1% Tween-20 in PBS, the plates were incubated in TMB solution (Life Technologies) for ~30 min in darkness before optical density (OD) signals were measured at 650 nm using a Synergy HTX plate reader (Biotek). Endpoint dilution titers were calculated by determining the highest dilution that gave an average OD 650 reading greater than or equal to the cut-off OD value. The cut-off value was set per plate and calculated as the mean OD value for all 0 DPV samples (at dilutions of 1:200 or 1:400, depending on the assay) plus three times the standard deviation of that mean. In instances where the endpoint titer was determined to lie below the lower limit of detection or above the upper limit of detection, arbitrary values of 100 and 583,200 were assigned, respectively. Data are depicted as the Log10 of the reciprocal endpoint dilution.
2.7. Plaque Reduction Neutralization Test (PRNT)
Lithium–heparin plasma samples from Experiment #1 or serum samples from Experiment #2 were obtained at 27 DPV, heat-inactivated (60 min at 56 °C), spun down at 15,000× g for 5 min, and subjected to a plaque reduction neutralization test (PRNT) using PHV01 FDS, which had undergone a single freeze/thaw cycle. Plasma or serum samples were serially diluted twofold (starting at 1:40) in DMEM. PHV01 FDS virus was then diluted in DMEM to 667 PFU/mL, and 200 µL was added to an equivalent volume of diluted plasma or serum, for a total volume of 400 µL containing 133 PFU virus and final plasma/serum concentrations of 1:80, 1:160, 1:320, 1:640, 1:1280, and 1:2560. The virus and plasma/serum mixture was incubated for 1 h at 37 °C, after which 150 µL (containing 50 PFU virus) was added to each well of a 12-well plate containing confluent monolayers of Vero E6 cells. Each dilution was tested in duplicate, and DMEM-only was used as a negative control. At 3 DPI, cells were fixed in 10% formalin containing crystal violet (0.5% w/v). Plaques were counted manually and the percent inhibition of infection was calculated by dividing the number of plaques in each test sample by the number of plaques in the negative control. The highest dilution that resulted in ≥50% inhibition of infection (PRNT50) was determined. In instances where the PRNT50 titer was determined to lie below the lower limit of detection (i.e., 80) or above the upper limit of detection (i.e., 2560), arbitrary values of 40 and 5120 were assigned, respectively. Data are depicted as the Log10 of the PRNT50 titer.
2.8. Statistical Analyses
All graphs were generated using GraphPad Prism version 9; all statistical tests were performed using the same software. Statistical comparisons of the Kaplan–Meier survival curves were performed using the Log-Rank test with the Bonferroni correction for multiple comparisons. The ordinary one-way ANOVA test and Tukey’s multiple comparison test were used to compare group means or geometric means against all other group means within Experiment #1, Experiment #2, or the pooled data. P-values less than or equal to 0.05 were marked with one asterisk (*), less than or equal to 0.01 were marked with two asterisks (**), less than or equal to 0.001 were marked with three asterisks (***), and less than or equal to 0.0001 were marked with four asterisks (****). Nonsignificant statistical comparisons are not labelled.
4. Discussion
A safe and effective vaccine capable of preventing MVD is urgently needed. Although MARV has been responsible for just over a dozen outbreaks over the past five decades, it remains a potentially serious threat to global public health, capable of causing severe disease and unpredictable epidemics. The recent report of MVD in Guinea exemplifies the ongoing threat that this virus poses to Africa, and circulation of the virus in bats throughout the continent [
26,
27,
28,
29,
30,
31], including in West Africa [
32], further underscores the risk to public health. Moreover, past instances of travelers returning home with MVD demonstrate the possibility of international spread [
33,
34]. While many experimental MARV vaccines have been developed over the last several years, only a few candidates have been evaluated in small Phase I clinical trials and none have been approved for use in humans [
8,
35].
In this study, we evaluated the protective efficacy of the live-attenuated PHV01 candidate vaccine, which expresses the MARV/Ang GP in a recombinant VSV backbone in place of the VSV G gene. Importantly, we evaluated both uncloned (rVSVΔG-MARV P3) and biologically cloned (PHV01 MVS and PHV01 FDS) viruses at different passage levels in an effort to not only confirm efficacy against GPA-MARV/Ang in the guinea pig model of infection, but also to confirm the reproducible efficacy of a clonally derived vaccine candidate, PHV01 FDS, produced via a manufacturing process representative of the GMP process that will be used to produce clinical material. Our results demonstrate that both PHV01 FDS and PHV01 MVS are highly effective, offering 100% protection at the highest dose levels tested and ~97% protection over the medium and high dose levels combined. Together, PHV01 MVS and FDS elicited significant IgG and neutralizing antibody responses that reduced or eliminated MARV replication in most vaccinated animals. These conclusions were not unexpected given previous studies performed with rVSVΔG-MARV/Ang and rVSVΔG-MARV/Mus in NHPs [
12,
13,
17,
18,
19,
24,
25]. Nevertheless, this study represents the first published evaluation of an rVSV-based MARV vaccine in a rodent, and it attests to the predictive value of the MARV guinea pig model in countermeasure evaluation.
The humoral immune response is thought to be essential to the immune protection conferred against EBOV by the rVSVΔG-EBOV vaccine [
36], and the same appears true for PHV01. All prior evaluations of rVSVΔG-MARV in NHPs demonstrated moderate to high levels of MARV GP-specific IgG response following vaccination [
12,
13,
17,
18,
24,
25], and the present study recapitulates these results in the guinea pig model. Almost all the vaccinated guinea pigs exhibited high levels of IgG at 27 DPV, and this was particularly evident in the animals that received the high and medium doses. Neutralizing activity was also detected in plasma/serum samples from most vaccinated animals, although the individual titers varied widely within each group and some surviving animals had no detectable levels of neutralizing activity. Interestingly, high levels of neutralizing antibodies have not been consistently identified in NHPs vaccinated with rVSVΔG-MARV [
12,
13,
17], suggesting that non-neutralizing antibodies may contribute to immunity through various other effector functions [
37]. Work with EBOV GP-based vaccines using the human parainfluenza virus or adenovirus/MVA platforms has demonstrated that Fc-mediated antibody effector functions contribute important immune protection in NHPs and humans, respectively [
38,
39]. Likewise, survivors of Ebola virus disease have been shown to develop polyfunctional antibodies capable of mediating a variety of Fc-mediated effector functions [
40]. Whether similar immune responses are elicited by PHV01 is currently unclear, but a recent study evaluating a different rVSVΔG-MARV/Ang vaccine in NHPs corroborated the induction of strong humoral immunity and described extensive transcriptional changes associated with innate immune activation and B- and T-cell proliferation, suggesting potential roles for other immune components [
18].
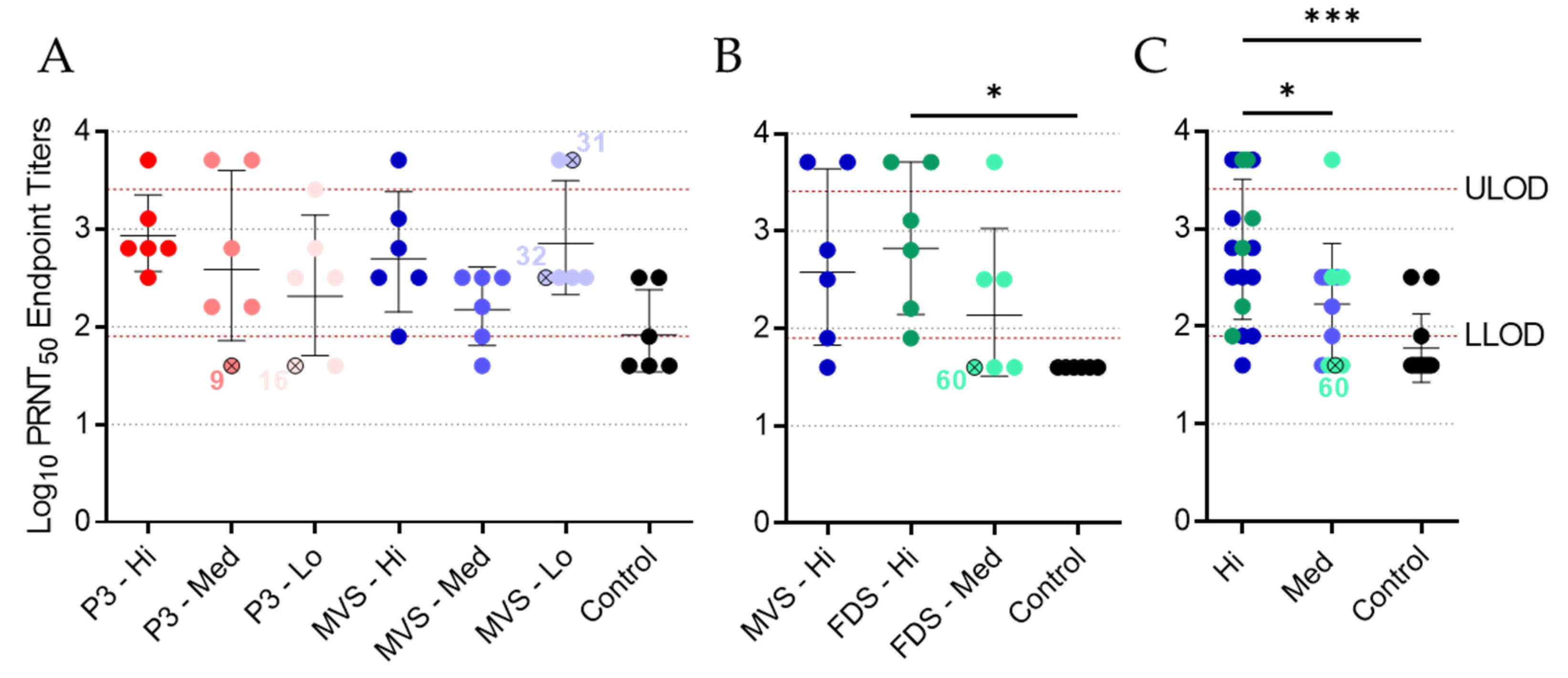
It is difficult to conclude whether the immunity elicited by PHV01 in this study was sterilizing, since we only quantified MARV infection via RT-qPCR at a single time point (i.e., 5 DPI) during acute disease. Nevertheless, no MARV RNA was detected in the vast majority of animals that received the high and medium doses of vaccine. Within these groups, virus RNA was undetectable in all but three animals—two of which ended up succumbing to disease. One of the non-survivors (animal 9; rVSV-MARV P3 medium-dose group) did not receive the full dose of vaccine, while the other non-survivor (animal 60; PHV01 FDS medium-dose group) may not have responded to vaccination at all, since no IgG or neutralizing antibody response was detected and only very low levels of vaccinemia were detected after immunization. The third animal—a survivor from the PHV01 FDS high-dose group—exhibited low levels of MARV RNA, although it is unclear how this relatively low RNA level correlates with infectious virus load. In any case, these results are in line with what has been observed in NHPs, where infectious MARV has never been reported in vaccinated animals [
12,
13,
17,
18,
24,
25]. At the low vaccine doses, we did detect high levels of MARV RNA in a few guinea pigs, all of which eventually succumbed to disease. Animals 16 and 32 exhibited low levels of MARV GP-specific IgG at 27 DPV, and either no or low levels of neutralizing antibodies, respectively, suggesting that the immune response was not sufficient to prevent disease. Conversely, animal 31 exhibited moderate levels of IgG and high levels of neutralizing activity despite eventually meeting the humane endpoint at 14 DPI, a week after all other non-survivors. It is not clear what caused the protracted disease in this animal, but we suspect that the outbred nature of these guinea pigs may introduce host-specific factors capable of influencing the animal’s response to vaccination or MARV infection. Alternatively, the virus may have acquired mutations that permitted it to escape the immune response elicited by vaccination.
In summary, our study demonstrated for the first time the protective efficacy of a VSV-based MARV GP vaccine in guinea pigs through a single-dose immunization. rVSVΔG-MARV P3 and PHV01 provided high survival rates against homologous MARV/Ang challenge, protecting 100% of the animals at the highest dose level tested and offering significant protection even at very low dose levels. In addition, our findings in guinea pigs aligned closely with the findings from NHP studies, including a recent study demonstrating PHV01 confers 100% protection against death, clinical signs of disease, and characteristic changes in clinical chemistry parameters in cynomolgus macaques challenged with a uniformly lethal dose of MARV/Ang (Shannan Rossi, personal communications). In light of the results presented here, this NHP study further highlights the utility of the guinea pig model of MARV infection as a bridge to NHPs.
Importantly, this study also specifically confirms the efficacy of the selected PHV01 FDS clonal vaccine candidate, with no significant difference observed in the degree of protection afforded by the uncloned and cloned materials. Furthermore, these findings validate the fidelity of the production process. PHV01 FDS was produced via a manufacturing process representative of the GMP process being used to produce vaccine material for clinical trials and regulated nonclinical testing. As demonstrated here, this process preserved MARV/Ang GP and its critical functional attributes through multiple passage levels. Notably, although PHV01 FDS, itself, was not sequence-confirmed, viruses from multiple passage levels preceding PHV01 FDS (including PHV01 MVS) displayed remarkable sequence fidelity, as did virus that was passaged three times beyond PHV01 MVS (
Figure S1), highlighting the stability of the rVSV genome. Moreover, the immune responses to all vaccine materials were similar, suggesting no impactful divergence among the three viruses. Even though the present study was not conducted under GLP conditions, the results bridge the efficacy observed in previous NHP studies using research-grade vaccine candidates with efficacy observed in a well-characterized guinea pig model using a vaccine candidate ready for clinical production. Together, these results provide additional evidence to advance preclinical and clinical development of this important human vaccine candidate.