An Absence of Epstein–Barr Virus Reactivation and Associations with Disease Activity in People with Multiple Sclerosis Undergoing Therapeutic Hookworm Vaccination
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Detection of EBV Antibodies
2.3. Statistical Methods
3. Results
3.1. Demographics of PwMS Groups
3.2. EBV VCA IgM Seroprevalence in the Hookworm- and Placebo-Treated Groups
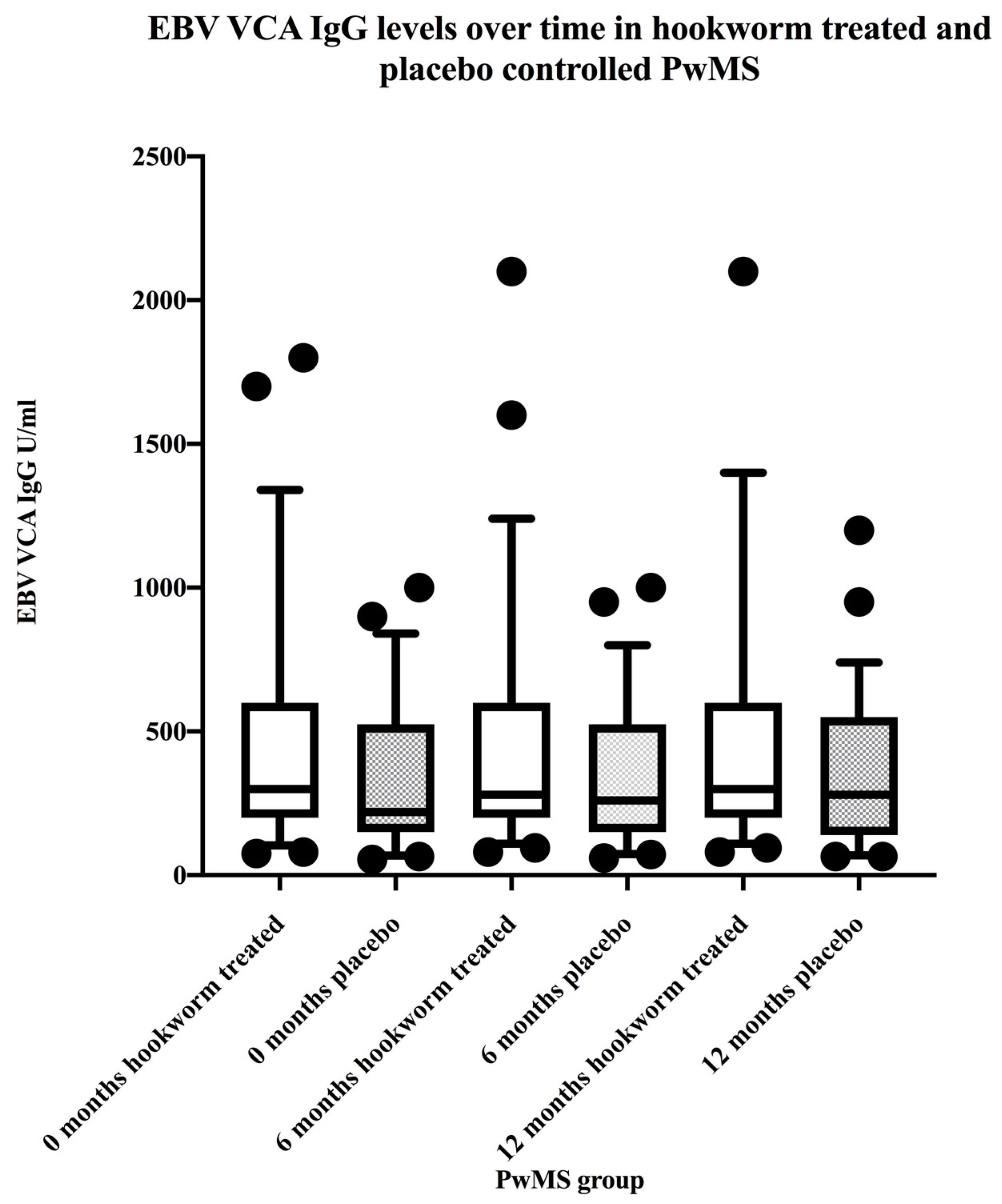
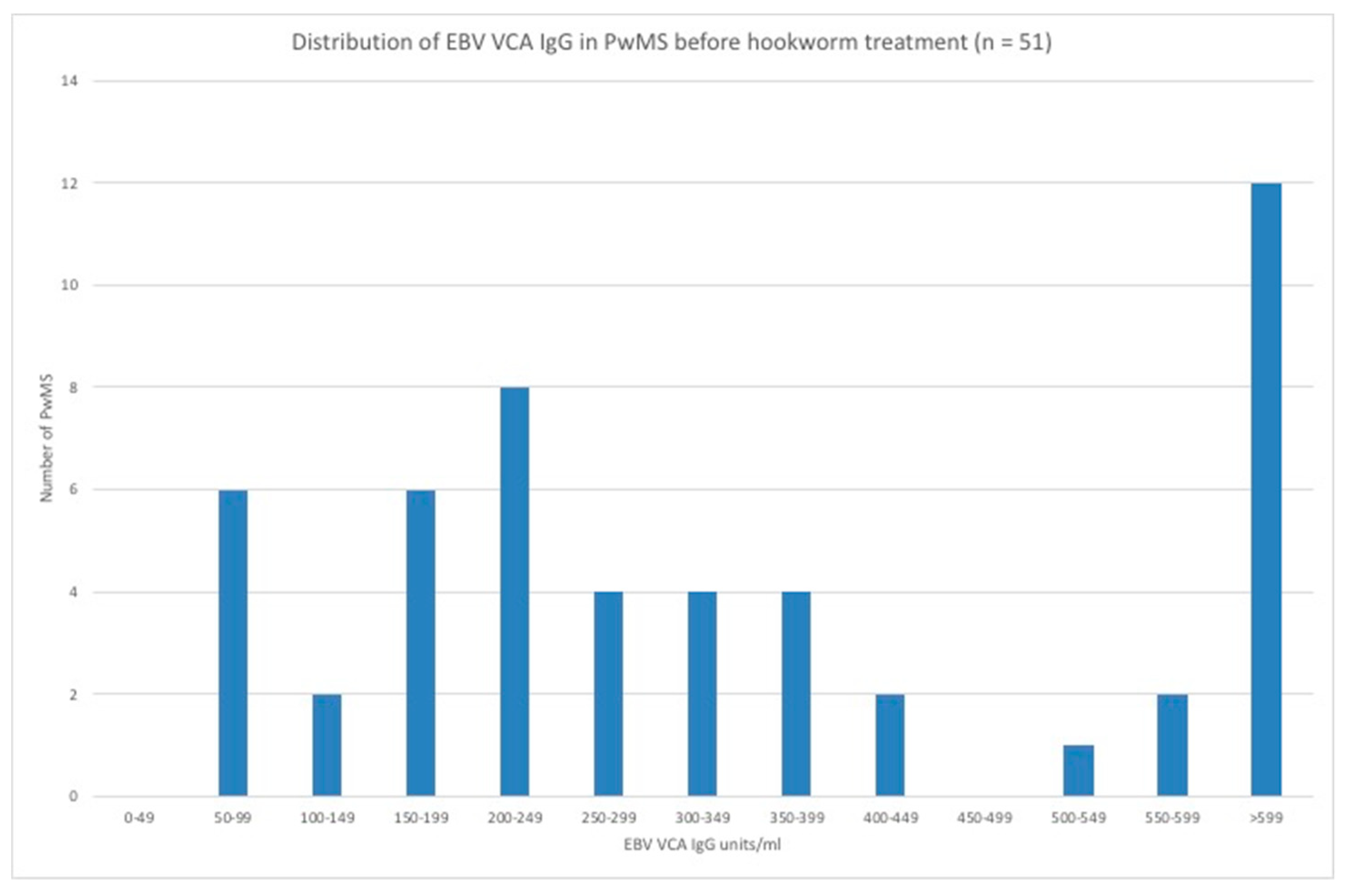
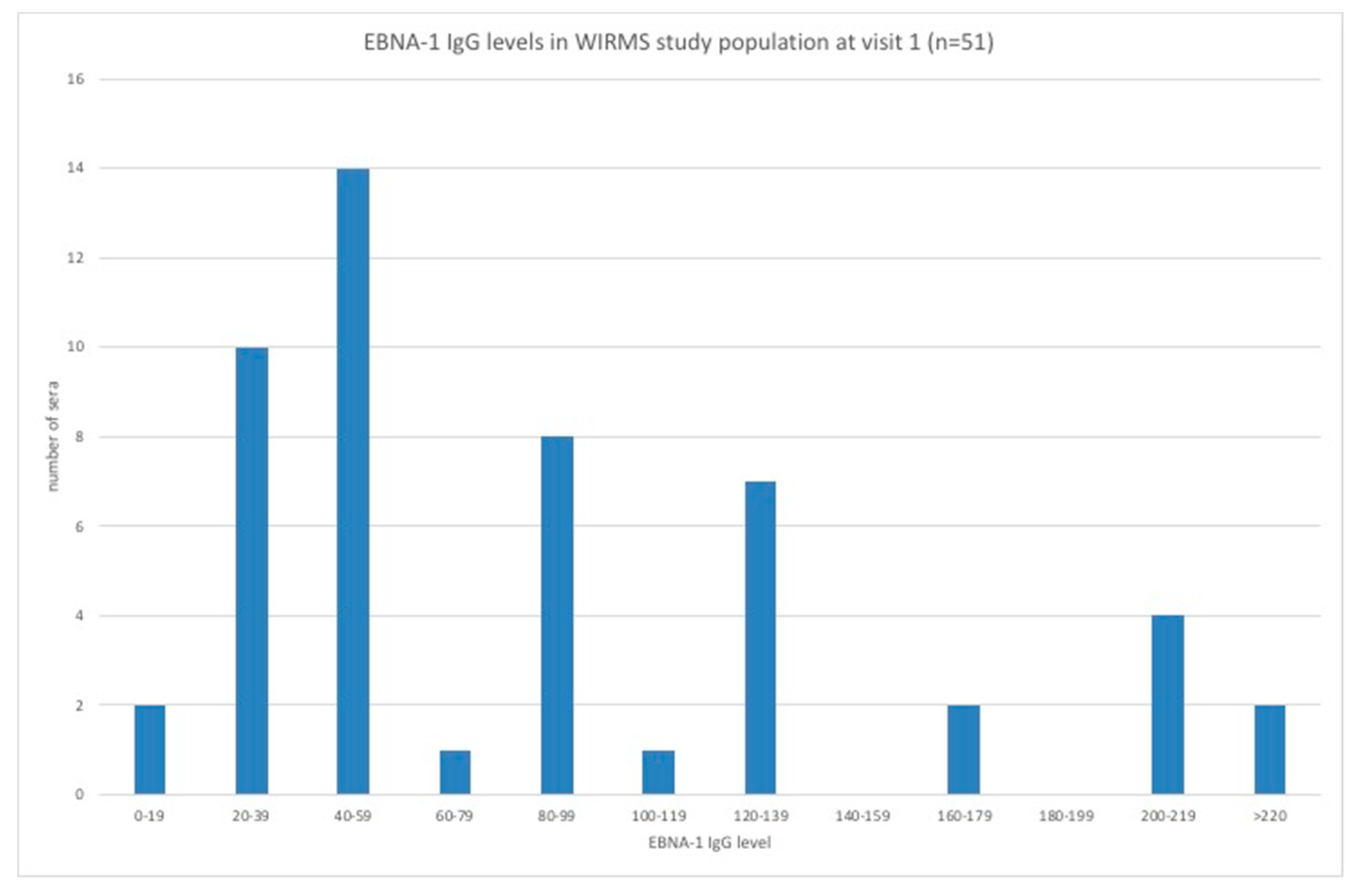
3.3. EBV VCA IgG and EBNA-1 IgG Seroprevalence and Levels in the Hookworm- and Placebo-Treated Groups
3.4. EBV EA IgG Seroprevalence and EBV Reactivation in the Hookworm- and Placebo-Treated Groups
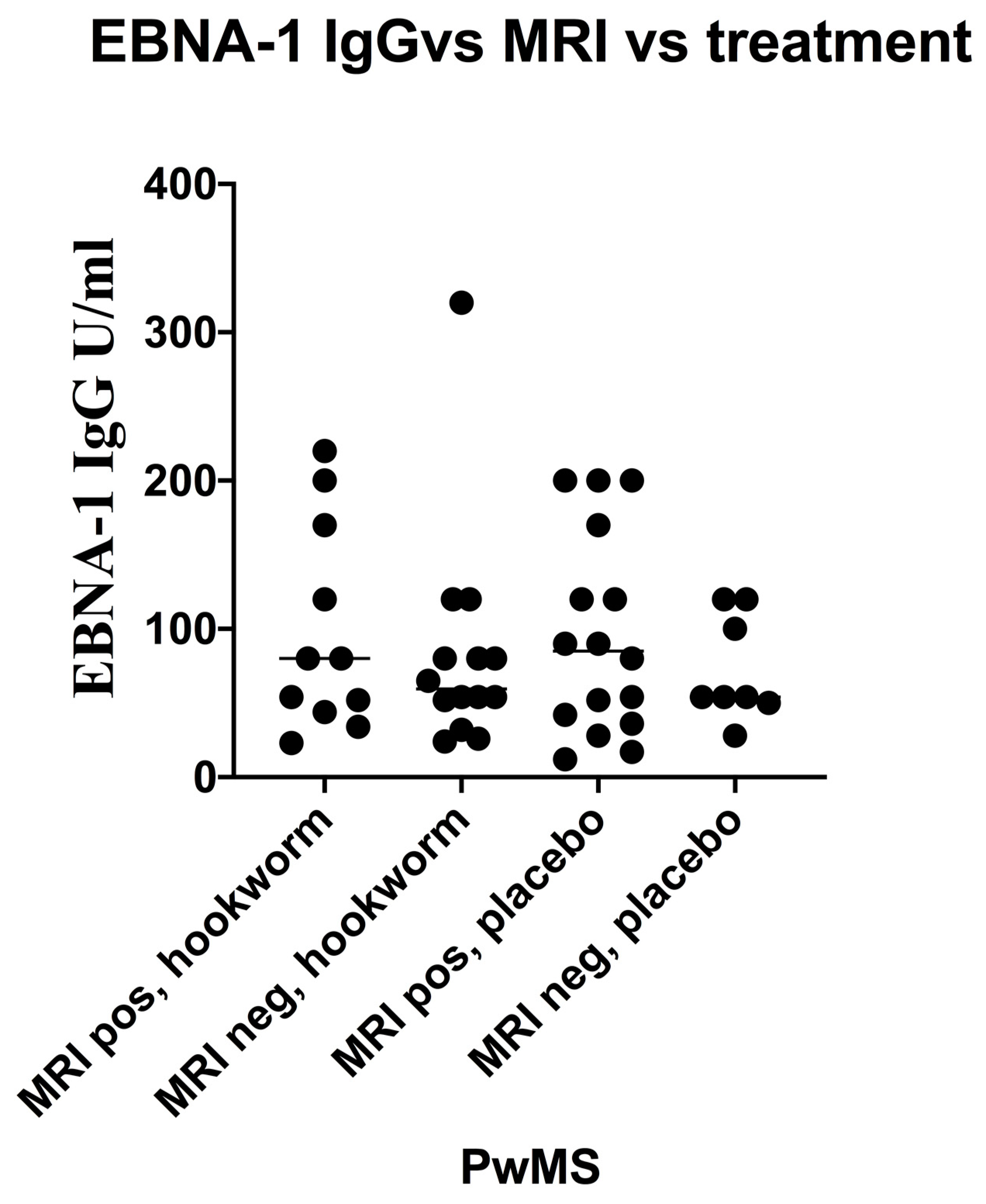
3.5. Stratification of Levels of EBV VCA IgG and EBNA-1 IgG and Association with Disease Activity in PwMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Bethony, J.; Bottazzi, M.E.; Brooker, S.; Buss, P. Hookworm: “the great infection of mankind”. PLoS Med. 2005, 2, e67. [Google Scholar] [CrossRef] [Green Version]
- Hotez, P. Hookworm and poverty. Ann. N. Y. Acad. Sci. 2008, 1136, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Brooker, S.; Bethony, J.M.; Bottazzi, M.E.; Loukas, A.; Xiao, S. Hookworm infection. N. Engl. J. Med. 2004, 351, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McSorley, H.J.; Maizels, R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiria, A.E.; Djuardi, Y.; Supali, T.; Sartono, E.; Yazdanbakhsh, M. Helminth infection in populations undergoing epidemiological transition: A friend or foe? Semin. Immunopathol. 2012, 34, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 78, 169–180. [Google Scholar] [CrossRef]
- Fleming, J.O.; Cook, T.D. Multiple sclerosis and the hygiene hypothesis. Neurology 2006, 67, 2085–2086. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth immodulation in autoimmune disease. Front. Immunol. 2017, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Fleming, J.O. Helminth therapy and multiple sclerosis. Int. J. Parasitol. 2013, 43, 259–274. [Google Scholar] [CrossRef]
- Osborne, L.C.; Monticelli, L.A.; Nice, T.J.; Sutherland, T.E.; Siracusa, M.C.; Hepworth, M.R.; Tomov, V.T.; Kobuley, D.; Tran, S.V.; Bittinger, K.; et al. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science 2014, 345, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Reese, T.A.; Wakeman, B.S.; Choi, H.S.; Hufford, M.M.; Huang, S.C.; Zhang, X.; Buck, M.D.; Jezewski, A.; Kambal, A.; Liu, C.Y.; et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 2014, 345, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Kalla, M.; Hammerschmidt, W. Human B cells on their route to latent infection–early but transient expression of lytic genes of Epstein-Barr virus. Eur. J. Cell. Biol. 2012, 91, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Weidner-Glunde, M.; Kruminis-Kaszkiel, E.; Savanagouder, M. Herpesviral latency-common themes. Pathogens 2020, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klutts, J.S.; Ford, B.A.; Perez, N.R.; Gronowski, A.M. Evidence-based approach for interpretation of Epstein-Barr virus serological patterns. J. Clin. Microbiol. 2009, 47, 3204–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, L.I.; Munger, K.L.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann. Neurol. 2010, 67, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandinger, K.; Jabs, W.; Siekhaus, A.; Bubel, P.; Trillenberg, H.-J.; Wagner, K.; Wessel, H.; Kirchner, H.H. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 2000, 55, 178–184. [Google Scholar] [CrossRef]
- Buljevac, D.; van Doornum, G.J.J.; Flach, H.Z.; Groen, J.; Osterhaus, A.D.M.E.; Hop, W.; Doorn, P.A.V.; Meché, F.G.A.V.D.; Hintzen, R.Q. Epstein-Barr virus and disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1377–1381. [Google Scholar] [CrossRef] [Green Version]
- Farrell, R.A.; Antony, D.; Wall, G.R.; Clark, L.; Fisniku, J.; Swanton, Z.; Khaleeli, K.; Schmierer, D.H.; Miller, G.G. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 2009, 73, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Kvistad, S.; Myhr, K.M.; Holmøy, T.; Bakke, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Løken-Amsrud, K.I.; Lilleås, F.; Midgard, R.; et al. Antibodies to Epstein-Barr virus and MRI disease activity in multiple sclerosis. Mult. Scler. 2014, 20, 1833–1840. [Google Scholar]
- Tanasescu, R.; Tench, C.R.; Constantinescu, C.S.; Telford, G.; Singh, S.; Frakich, N.; Onion, D.; Dorothee, P.A.; Gran, B.; Evangelou, N.; et al. Hookworm treatment for relapsing multiple sclerosis a randomized double-blinded placebo-controlled trial. JAMA Neurol. 2020, 15, e201118. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sorensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis. The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar]
- Zozulya, A.L.; Wiendl, H. The role of regulatory T cells in multiple sclerosis. Nat. Clin. Pract. Neurol. 2008, 4, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Staun-Ram, E.; Miller, A. Effector and regulatory B cells in multiple sclerosis. Clin. Immunol. 2017, 18, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Scorsi, E.; Rosicarelli, B.; Rigau, V.; Thouvenot, E.; Aloisi, F. Massive intracerebral Epstein-Barr virus reactivation in lethal multiple sclerosis relapse after Natalizumab withdrawal. J. Neuroimmunol. 2017, 307, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.D. Routine Epstein-Barr virus diagnostics from the laboratory perspective: Still challenging after 35 years. J. Clin. Microbiol. 2004, 42, 3381–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abusalah, M.A.; Gan, S.H.; Al-Hatamleh, M.A.; Irekeola, A.A.; Shueb, R.H.; Yean, C.Y. Recent advances in diagnostic approaches for Epstein-Barr virus. Pathogens 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smatti, M.K.; Yassine, H.M.; AbuOdeh, R.; AlMarawani, A.; Taleb, S.A.; Althani, A.A.; Nasrallah, G.K. Prevalence and molecular profiling of Epstein-Barr virus (EBV) among healthy blood donors from different nationalities in Qatar. PLoS ONE 2017, 12, e0189033. [Google Scholar] [CrossRef] [Green Version]
- Pandit, L.; Malli, C.; D’Cunha, A.; Shetty, R.; Singhal, B.B. Association of Epstein-Barr virus infection with multiple sclerosis in India. J. Neurol. Sci. 2013, 325, 86–89. [Google Scholar] [CrossRef]
- Maple, P.A.; Tanasescu, R.; Gran, B.; Constantinescu, C.S. A different response to cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infection in UK people with multiple sclerosis (PwMS) compared to controls. J. Infect. 2020, 80, 320–325. [Google Scholar] [CrossRef]
- Horakova, D.; Zivadinov, R.; Weinstock-Guttman, B.; Havrdova, E.; Qu, J.; Tamano-Blanco, M.; Badgett, D.; Tyblova, M.; Bergsland, N.; Hussein, S. Environmental factors associated with disease progression after the first demyelinating event: Results from the multi-center SET study. PLoS ONE 2013, 8, e53996. [Google Scholar] [CrossRef]
- Jakimovski, D.; Ramanathan, M.; Weinstock-Guttman, B.; Dwyer, M.; Bergsland, N.; Ramasamy, D.; Carl, E.; Zivadinov, R. Higher EBV response is associated with more severe gray matter and lesion pathology in relapsing multiple sclerosis patients: A case-controlled magnetization transfer ratio study. Mult. Scler. 2020, 26, 322–332. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Bethony, J.; Pritchard, D.I. The immunoepidemiology of human hookworm infection. Parasite Immunol. 2004, 26, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Pritchard, D.I.; Raiko, A.; Brown, A.P.; Shaw, M.A. Immune responses in human necatoriasis: Associations between interleukin-5 responses and resistance to reinfection. J. Infect. Dis. 2004, 190, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, D.I.; Brown, A. Is Necator americanus approaching a mutualistic symbiotic relationship with humans? Trends Parasitol. 2001, 17, 169–172. [Google Scholar] [CrossRef]
- Pritchard, D.I.; Quinnell, R.J.; Walsh, E.A. Immunity in humans to Necator americanus: IgE, parasite weight and fecundity. Parasite Immunol. 1995, 17, 71–75. [Google Scholar] [CrossRef]
- Pritchard, D.I.; Quinnell, R.J.; Moustafa, M.; McKean, P.G.; Slater, A.F.G.; Raiko, A.; Dale, D.D.S.; Keymer, A.E. Hookworm (Necator americanus) infection and storage iron depletion. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 235–238. [Google Scholar] [CrossRef]
- Hotez, P.J.; Pritchard, D.I. Hookworm infection. Sci. Am. 1995, 27, 68–74. [Google Scholar] [CrossRef]
- McSorley, H.I.; Loukas, A. The immunology of human hookworm infections. Parasite Immunol. 2010, 32, 549–559. [Google Scholar] [CrossRef]


| Patient Characteristics | Hookworm | Placebo |
|---|---|---|
| Demographic characteristics | ||
| Age | ||
| (mean age, range) | 44.8 (32–60) | 49.3 (29–59) |
| Sex (no. of subjects, %) | ||
| Female | 21 (81%) | 19 (76%) |
| Male | 5 (19%) | 6 (24%) |
| Clinical characteristics | ||
| Type of relapsing MS | ||
| (no. of subjects, %) | 21 (81%) | 21 (84%) |
| RR | ||
| SP with superimposed relapses | 5 (19%) | 4 (16%) |
| Time from first symptoms of MS (mean years, SD) | 11 (8.3) | 9.2 (9.3) |
| Mean EDSS score (range) | 3 (1.5–5.5) | 2.6 (1.5–4) |
| EBV Antibody and Treatment Group | Before Treatment (0 months) | During Treatment (6 months) | Post Treatment (12 months) |
|---|---|---|---|
| EBV VCA IgG | 340.2 units/mL | 347.2 units/mL | 351.1 units/mL |
| Hookworm treated | 95% CI: [242–476.3] | 95% CI: [248–486] | 95% CI: [250–493] |
| EBV VCA IgG | 251.4 units/mL | 257.0 units/mL | 254.8 units/mL |
| Placebo | 95% CI: [179–352] | 95% CI: [185–355] | 95% CI: [183–354] |
| EBNA-1 IgG | 67.8 units/mL | 64.7 units/mL | 65.7 units/mL |
| Hookworm treated | 95% CI: [50.2–91.6] | 95% CI: [45.9–91.3] | 95% CI: [46.6–92.6] |
| EBNA-1 IgG | 66.4 units/mL | 65.3 units/mL | 67.0 units/mL |
| Placebo | 95% CI: [48.6–90.9] | 95% CI: [49–86.8] | 95% CI: [50.4–89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maple, P.A.C.; Gran, B.; Tanasescu, R.; Pritchard, D.I.; Constantinescu, C.S. An Absence of Epstein–Barr Virus Reactivation and Associations with Disease Activity in People with Multiple Sclerosis Undergoing Therapeutic Hookworm Vaccination. Vaccines 2020, 8, 487. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines8030487
Maple PAC, Gran B, Tanasescu R, Pritchard DI, Constantinescu CS. An Absence of Epstein–Barr Virus Reactivation and Associations with Disease Activity in People with Multiple Sclerosis Undergoing Therapeutic Hookworm Vaccination. Vaccines. 2020; 8(3):487. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines8030487
Chicago/Turabian StyleMaple, Peter A. C., Bruno Gran, Radu Tanasescu, David I. Pritchard, and Cris S. Constantinescu. 2020. "An Absence of Epstein–Barr Virus Reactivation and Associations with Disease Activity in People with Multiple Sclerosis Undergoing Therapeutic Hookworm Vaccination" Vaccines 8, no. 3: 487. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines8030487





