COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform
Abstract
:1. Introduction
2. Discovery/History of Coronavirus
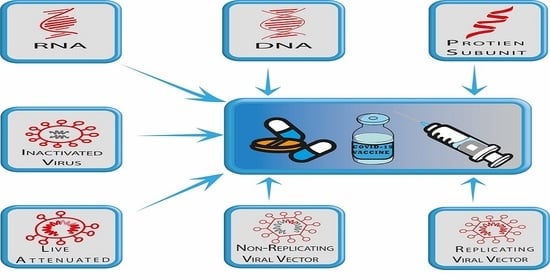
3. COVID-19 (SARS-Cov-2) Vaccine Platforms
3.1. Inactivated or Killed Vaccines
3.2. Live-Attenuated Vaccines
3.3. Recombinant Vaccines
3.4. Nucleic Acid-Based Vaccines
4. SARS-CoV-2 (COVID-19) Vaccines in Clinical Trials (Phase I–III)
4.1. AstraZeneca’s ChAdOxnCoV-19 (AZD1222) Vaccine
4.2. Sinopharm’s BBIBP-CorV Vaccine
4.3. CanSino’s AdV5-Based Vaccine
4.4. Gamaleya’s Sputnik V (Gam-COVID-VacLyo) Vaccine
4.5. Novavax’ NVX-CoV2373
4.6. Sinovac’s CoronaVac (PiCoVacc)
4.7. Johnson & Johnson (J & J)’s Ad26.COV2.S (JNJ-78436735)
5. Licensed SARS-CoV-2 (COVID-19) Vaccines
5.1. Pfizer-BioNTech’s BNT162b2 Vaccine
5.2. Moderna’s mRNA-1273 Vaccine
6. Mucosal Vaccines-Platform for COVID-19 Vaccine Development
6.1. Vaxart’s Oral Mucosal COVID-19 Vaccine
6.2. IosBio’s (Sabilitech’s) OraPro-COVID-19™ Vaccine
6.3. Broad-Spectrum of Pre-Existing Mucosal Vaccines
6.4. RPS-Vector System as A Potential Platform for COVID-19 Oral Mucosal Vaccine
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Trilla, A.; Trilla, G.; Daer, C. The 1918 “Spanish Flu” in Spain. Clin. Infect. Dis. 2008, 47, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.V.V.; Thanh Lam, V.; Thanh Dung, N.; Yen, L.M.; Minh, N.N.Q.; Hung, L.M.; Ngoc, N.M.; Dung, N.T.; Man, D.N.H.; Nguyet, L.A.; et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Sungnak, W.; Network, H.L.B.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Sallenave, J.-M.; Guillot, L. Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front. Immunol. 2020, 11, 1229. [Google Scholar] [CrossRef]
- Zhou, R.; To, K.K.-W.; Wong, Y.-C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.-Y.; Lau, T.T.-K.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef] [PubMed]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.U.; Jeong, Y.; Roh, S.-E.; Bae, Y.-S. Transendothelial migration (TEM) of in vitro generated dendritic cell vaccine in cancer immunotherapy. Arch. Pharmacal Res. 2019, 42, 582–590. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.-S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Charles, T.P.; Joag, V.; Bollimpelli, V.S.; Scott, M.K.D.; Wimmers, F.; Burton, S.L.; Labranche, C.C.; Petitdemange, C.; Gangadhara, S.; et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 2020, 26, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. Immunopathology, host-virus genome interactions, and effective vaccine development in SARS-CoV-2. Comput. Struct. Biotechnol. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.D.; Han, I.; Choi, E.-H.; Yadav, D.K. Recent Advances in Pathophysiology, Drug Development and Future Perspectives of SARS-CoV-2. Front. Cell Dev. Biol. 2020, 8, 580202. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.P.; Sharma, A.R.; Singh, M.K.; Samanta, S.; Bhakta, S.; Mandal, S.; Bhattacharya, M.; Lee, S.-S.; Chakraborty, C. Repurposing Drugs, Ongoing Vaccine, and New Therapeutic Development Initiatives Against COVID-19. Front. Pharmacol. 2020, 11, 1258. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19). Available online: https://www.google.com/search?q=covid-19+fatality+rate+percentage&oq=Coronavirus-19+fatality+&aqs=chrome.2.69i57j0i10i22i30i457j0i22i30l5.14707j1j15&sourceid=chrome&ie=UTF-8 (accessed on 30 November 2020).
- Mortality Risk of COVID-19. Available online: https://ourworldindata.org/mortality-risk-covid (accessed on 30 November 2020).
- O’Leary, D.R.; Marfin, A.A.; Montgomery, S.P.; Kipp, A.M.; Lehman, J.A.; Biggerstaff, B.J.; Elko, V.L.; Collins, P.D.; Jones, J.E.; Campbell, G.L. The Epidemic of West Nile Virus in the United States, 2002. Vector-Borne Zoonotic Dis. 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Añez, G.; Rios, M. Dengue in the United States of America: A Worsening Scenario? BioMed Res. Int. 2013, 2013, 678645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, O.B.; Martín, J.L.S.; Del Diego, J.; Montoya, R.H.; Dayan, G.H.; Zambrano, B. The History of Dengue Outbreaks in the Americas. Am. J. Trop. Med. Hyg. 2012, 87, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Ligon, B.L. Outbreak of Marburg Hemorrhagic Fever in Angola: A Review of the History of the Disease and its Biological Aspects. Semin. Pediatr. Infect. Dis. 2005, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Marburg Haemorrhagic Fever in ANGOLA—Update. Available online: https://www.who.int/csr/don/2005_03_23/en/ (accessed on 25 December 2020).
- Renault, P.; Josseran, L.; Pierre, V. Chikungunya-related Fatality Rates, Mauritius, India, and Reunion Island. Emerg. Infect. Dis. 2008, 14, 1327. [Google Scholar] [CrossRef]
- Coltart, C.E.M.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola outbreak, 2013–2016: Old lessons for new epidemics. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160297. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Ospina, J.A.; Henao-SanMartin, V.; Acevedo-Mendoza, W.F.; Nasner-Posso, K.M.; Martínez-Pulgarín, D.F.; Restrepo-López, A.; Valencia-Gallego, V.; Collins, M.H.; Rodriguez-Morales, A.J.; Gallego-Valencia, V. Fatal Zika virus infection in the Americas: A systematic review. Int. J. Infect. Dis. 2019, 88, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possas, C.; Lourenço-De-Oliveira, R.; Tauil, P.L.; Pinheiro, F.D.P.; Pissinatti, A.; Da Cunha, R.V.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Memórias Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akpede, G.O.; Asogun, D.A.; Okogbenin, S.A.; Dawodu, S.O.; Momoh, M.O.; Dongo, A.E.; Ike, C.; Tobin, E.; Akpede, N.; Ogbaini-Emovon, E.; et al. Caseload and Case Fatality of Lassa Fever in Nigeria, 2001–2018: A Specialist Center’s Experience and Its Implications. Front. Public Health 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.K.; Jean, C.; Acosta, M.; Samuel, M.C.; Mátyás, B.T.; Schechter, R. A Review of Adult Mortality Due to 2009 Pandemic (H1N1) Influenza A in California. PLoS ONE 2011, 6, e18221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charu, V.; Chowell, G.; Mejia, L.S.P.; Echevarría-Zuno, S.; Borja-Aburto, V.H.; Simonsen, L.; Miller, M.A.; Viboud, C. Mortality Burden of the A/H1N1 Pandemic in Mexico: A Comparison of Deaths and Years of Life Lost to Seasonal Influenza. Clin. Infect. Dis. 2011, 53, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Fact Check: 2009 Swine Flu Spread Rapidly, But COVID-19 Is More Deadly. Available online: https://www.usatoday.com/story/news/factcheck/2020/08/13/fact-check-swine-flu-spread-rapidly-but-not-deadly-covid-19/5577001002/ (accessed on 26 December 2020).
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC7204879/ (accessed on 30 November 2020).
- Sizun, J.; Yu, M.; Talbot, P. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: A possible source ofhospital-acquired infections. J. Hosp. Infect. 2000, 46, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nat. Cell Biol. 2020, 586, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Vellozzi, C.; Burwen, D.R.; Dobardzic, A.; Ball, R.; Walton, K.; Haber, P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine 2009, 27, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdin, A.D.; Barreto, L.; Plotkin, S. Inactivated poliovirus vaccine: Past and present experience. Vaccine 1996, 14, 735–746. [Google Scholar] [CrossRef]
- Kusov, Y.; Elbert, L.; Nelga, I.; Grishina, G.; Dunaevski, O.; Kharin, N.; Maslov, Y.; Drozdov, S.; Balayan, M. Immunogenicity trial of inactivated hepatitis A virus vaccine in human volunteers. Vaccine 1991, 9, 540–541. [Google Scholar] [CrossRef]
- Furesz, J.; Scheifele, D.W.; Palkonyay, L. Safety and effectiveness of the new inactivated hepatitis A virus vaccine. Can. Med. Assoc. J. 1995, 152, 343–348. [Google Scholar]
- Wu, W.; Liu, D.; Li, K.; Nuorti, J.P.; Nohynek, H.M.; Xu, D.; Ye, J.; Zheng, J.; Wang, H. Post-marketing safety surveillance for inactivated and live-attenuated Japanese encephalitis vaccines in China, 2008–2013. Vaccine 2017, 35, 3666–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Qamar, M.T.U.; Saleem, S.; Ashfaq, U.A.; Bari, A.; Anwar, F.; Alqahtani, S. Epitope-based peptide vaccine design and target site depiction against Middle East Respiratory Syndrome Coronavirus: An immune-informatics study. J. Transl. Med. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, eabb9983. [Google Scholar] [CrossRef] [PubMed]
- Immunogenicity and Safety of a SARS-CoV-2 Inactivated Vaccine in Healthy Adults Aged 18–59 Years: Report of the Randomized, Double-Blind, and Placebo-Controlled Phase 2 Clinical Trial. Available online: https://www.medrxiv.org/content/10.1101/2020.07.31.20161216v1 (accessed on 19 November 2020).
- WHO. DRAFT Landscape of COVID-19 Candidate Vaccines. 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 19 November 2020).
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479-480, 379–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohn, K.G.-I.; Smith, I.; Sjursen, H.; Cox, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccines Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Talon, J.; Salvatore, M.; O’Neill, R.E.; Nakaya, Y.; Zheng, H.; Muster, T.; García-Sastre, A.; Palese, P. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc. Natl. Acad. Sci. USA 2000, 97, 4309–4314. [Google Scholar] [CrossRef] [Green Version]
- Broadbent, A.J.; Santos, C.P.; Anafu, A.; Wimmer, E.; Mueller, S.; Subbarao, K. Evaluation of the attenuation, immunogenicity, and efficacy of a live virus vaccine generated by codon-pair bias de-optimization of the 2009 pandemic H1N1 influenza virus, in ferrets. Vaccine 2016, 34, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Nieto-Torres, J.L.; DeDiego, M.L.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Perlman, S.; Enjuanes, L. Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine. PLOS Pathog. 2015, 11, e1005215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, P.N.; Mundt, W.; Kistner, O.; Howard, M.K. Vero cell platform in vaccine production: Moving towards cell culture-based viral vaccines. Expert Rev. Vaccines 2009, 8, 607–618. [Google Scholar] [CrossRef]
- Live attenuated influenza vaccine for children. Drug Ther. Bull. 2017, 55, 114–117. [CrossRef]
- Armitage, E.P.; Camara, J.; Bah, S.; Forster, A.S.; Clarke, E.; Kampmann, B.; De Silva, T.I. Acceptability of intranasal live attenuated influenza vaccine, influenza knowledge and vaccine intent in The Gambia. Vaccine 2018, 36, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Bahamondez-Canas, T.F.; Cui, Z. Intranasal immunization with dry powder vaccines. Eur. J. Pharm. Biopharm. 2018, 122, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lim, A.; Alonso, S. AttenuatedBordetella pertussisBPZE1 as a live vehicle for heterologous vaccine antigens delivery through the nasal route. Bioeng. Bugs 2011, 2, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Montinaro, V.; Groppali, E.; Tenconi, R.; Semino, M.; Principi, N. Live attenuated intranasal influenza vaccine. Hum. Vaccines Immunother. 2012, 8, 76–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 2020, 10, 5932–5942. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, W.; Kubota, N.; Shimizu, T.; Saruta, J.; Fuchida, S.; Kawata, A.; Yamamoto, Y.; Sugimoto, M.; Yakeishi, M.; Tsukinoki, K. Existence of SARS-CoV-2 Entry Molecules in the Oral Cavity. Int. J. Mol. Sci. 2020, 21, 6000. [Google Scholar] [CrossRef]
- Gurwith, M.; Condit, R.C.; Excler, J.-L.; Robertson, J.S.; Kim, D.; Fast, P.E.; Drew, S.; Wood, D.; Klug, B.; Whelan, M.; et al. Brighton Collaboration Viral Vector Vaccines Safety Working Group (V3SWG) standardized template for collection of key information for benefit-risk assessment of live-attenuated viral vaccines. Vaccine 2020, 38, 7702–7707. [Google Scholar] [CrossRef] [PubMed]
- Halsey, N.A.; Talaat, K.R.; Greenbaum, A.; Mensah, E.; Dudley, M.Z.; Proveaux, T.; Salmon, D.A. The safety of influenza vaccines in children: An Institute for Vaccine Safety white paper. Vaccine 2015, 33, F1–F67. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Diaz-Arévalo, D.; Guan, H.; Zeng, M. Noninvasive vaccination against infectious diseases. Hum. Vaccines Immunother. 2018, 14, 1717–1733. [Google Scholar] [CrossRef]
- Bhandari, R.; Khanna, G.; Kuhad, A. Pharmacological insight into potential therapeutic agents for the deadly Covid-19 pandemic. Eur. J. Pharmacol. 2021, 890, 173643. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Jang, Y. Cold-Adapted Live Attenuated SARS-Cov-2 Vaccine Completely Protects Human ACE2 Transgenic Mice from SARS-Cov-2 Infection. Vaccines 2020, 8, 584. [Google Scholar] [CrossRef]
- Sims, A.C.; Baric, R.S.; Yount, B.; Burkett, S.E.; Collins, P.L.; Pickles, R.J. Severe Acute Respiratory Syndrome Coronavirus Infection of Human Ciliated Airway Epithelia: Role of Ciliated Cells in Viral Spread in the Conducting Airways of the Lungs. J. Virol. 2005, 79, 15511–15524. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, I.; Leite, L. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- Mardanova, E.S.; Ravin, N.V. Plant-produced Recombinant Influenza a Vaccines Based on the M2e Peptide. Curr. Pharm. Des. 2018, 24, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.T. Recombinant vaccines. Expert Rev. Vaccines 2010, 9, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, X.; Liu, J.; Jiang, S. Protocol for Recombinant RBD-based SARS Vaccines: Protein Preparation, Animal Vaccination and Neutralization Detection. J. Vis. Exp. 2011, 10, e2444. [Google Scholar] [CrossRef] [Green Version]
- Huber, V.C. Influenza vaccines: From whole virus preparations to recombinant protein technology. Expert Rev. Vaccines 2013, 13, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2017, 153, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Draper, S.J.; Heeney, J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Genet. 2009, 8, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Hollister, J.R. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 2009, 37, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Palm, A.-K.E.; Utset, H.A.; Huang, M.; Ho, I.Y.; Zheng, N.-Y.; Fitzgerald, T.; Neu, K.E.; Chen, Y.-Q.; Krammer, F.; et al. Monoclonal Antibody Responses after Recombinant Hemagglutinin Vaccine versus Subunit Inactivated Influenza Virus Vaccine: A Comparative Study. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, M.M.; Patriarca, P.A.; Treanor, J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influ. Other Respir. Viruses 2008, 2, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; He, Y.; Shen, B. Ontology-Based Vaccine Adverse Event Representation and Analysis. Adv. Exp. Med. Biol. 2017, 1028, 89–103. [Google Scholar] [CrossRef]
- Cox, M.M.J.; Izikson, R.; Post, P.; Dunkle, L.M. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther. Adv. Vaccines 2015, 3, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Mirza, F.; Pasquetto, V.; Tscharke, D.C.; Palmowski, M.J.; Dunbar, P.R.; Sette, A.; Harris, A.L.; Cerundolo, V. Immunodominance of Poxviral-Specific CTL in a Human Trial of Recombinant-Modified Vaccinia Ankara. J. Immunol. 2005, 175, 8431–8437. [Google Scholar] [CrossRef] [Green Version]
- Thomson, S.A.; Elliott, S.L.; Sherritt, M.A.; Sproat, K.W.; Coupar, B.E.; Scalzo, A.A.; Forbes, C.A.; Ladhams, A.M.; Mo, X.Y.; Tripp, R.A.; et al. Recombinant polyepitope vaccines for the delivery of multiple CD8 cytotoxic T cell epitopes. J. Immunol. 1996, 157, 822–826. [Google Scholar]
- Smith, C.L.; Dunbar, P.R.; Mirza, F.; Palmowski, M.J.; Shepherd, D.; Gilbert, S.C.; Coulie, P.; Schneider, J.; Hoffman, E.; Hawkins, R.; et al. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int. J. Cancer 2004, 113, 259–266. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, W.; Xia, S.; Gu, C.; Wang, X.; Wang, Q.; Zhou, J.; Wu, Y.; Cai, X.; Qu, D.; et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct. Target. Ther. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Zheng, T.; Xu, K.; Han, Y.; Xu, L.; Huang, E.; An, Y.; Cheng, Y.; Li, S.; Liu, M.; et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722–733.e11. [Google Scholar] [CrossRef]
- Ravichandran, S.; Coyle, E.M.; Klenow, L.; Tang, J.; Grubbs, G.; Liu, S.; Wang, T.; Golding, H.; Khurana, S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci. Transl. Med. 2020, 12, eabc3539. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K. Vaccine Against Covid-19 Disease—Present Status of Development. Indian J. Pediatr. 2020, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.R.; Sarver, N. Nucleic acid vaccines. Clin. Microbiol. Rev. 1995, 8, 406–410. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nat. Cell Biol. 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H.; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Lu, J.; Lu, G.; Tan, S.; Xia, J.; Xiong, H.; Yu, X.; Qi, Q.; Yu, X.; Li, L.; Yu, H.; et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020, 30, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [Green Version]
- Schnee, M.; Vogel, A.B.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An mRNA Vaccine Encoding Rabies Virus Glycoprotein Induces Protection against Lethal Infection in Mice and Correlates of Protection in Adult and Newborn Pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- FDA Takes Additional Action in Fight against COVID-19 by Issuing Emergency Use Authorization for Second COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid (accessed on 25 December 2020).
- Mahase, E. Covid-19: Pfizer and BioNTech submit vaccine for US authorisation. BMJ 2020, 371, m4552. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Explained: Why RNA Vaccines for Covid-19 Raced to the Front of the Pack. Available online: https://news.mit.edu/2020/rna-vaccines-explained-covid-19-1211 (accessed on 26 December 2020).
- Zeng, C.; Hou, X.; Yan, J.; Zhang, C.; Li, W.; Zhao, W.; Du, S.; Dong, Y. Leveraging mRNAs Sequences to Express SARS-CoV-2 Antigens in vivo. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mutant Coronavirus in the United Kingdom Sets Off Alarms, But Its Importance Remains Unclear. Available online: https://www.sciencemag.org/news/2020/12/mutant-coronavirus-united-kingdom-sets-alarms-its-importance-remains-unclear (accessed on 26 December 2020).
- Arcturus Therapeutics and Duke-NUS Medical School Partner to Develop a Coronavirus (COVID-19) Vaccine using STARR™ Technology. Available online: https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-therapeutics-and-duke-nus-medical-school-partner (accessed on 26 December 2020).
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Zeng, C.; Hou, X.; Yan, J.; Zhang, C.; Li, W.; Zhao, W.; Du, S.; Dong, Y. Leveraging mRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens In Vivo. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- CanSino’s Coronavirus Vaccine Candidate Approved for Military Use in China. Available online: https://www.cnbc.com/2020/06/29/cansinos-coronavirus-vaccine-candidate-approved-for-military-use-in-china.html (accessed on 26 November 2020).
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; Mcmahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Lowery, D. mRNA vaccines: Intellectual property landscape. Nat. Rev. Drug Discov. 2020, 19, 578. [Google Scholar] [CrossRef]
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Sah, R.; Al-Tawfiq, J.A.; Al-Qaaneh, A.M.; Al-Jamea, L.H.; Woodman, A.; Al-Qahtani, M.; Haque, S.; Harapan, H.; et al. Recent advances in vaccine and immunotherapy for COVID-19. Hum. Vaccines Immunother. 2020, 16, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Sayedahmed, E.E.; Elkashif, A.; Alhashimi, M.; Sambhara, S.; Mittal, S.K. Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines. Vaccines 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.R.; Gilbride, C.; Allen, E.; Belij-Rammerstorfer, S.; Bissett, C.; Ewer, K.; Lambe, T. The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world. Immunology 2020, 160, 223–232. [Google Scholar] [CrossRef]
- Poland, A.G.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- AstraZeneca’s COVID-19 Vaccine Authorised in Five Other Countries. Available online: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/serum-institute-of-india-obtains-emergency-use-authorisation-in-india-for-astrazenecas-covid-19-vaccine.html (accessed on 20 January 2021).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- China Injects Hundreds of Thousands With Experimental Covid-19 Vaccines. Available online: https://www.wsj.com/articles/china-injects-hundreds-of-thousands-with-experimental-covid-19-vaccines-11599834029 (accessed on 25 December 2020).
- Russia’s Claim of a Successful COVID-19 Vaccine Doesn’t Pass the ‘Smell Test,’ Critics Say. Available online: https://www.sciencemag.org/news/2020/11/russia-s-claim-successful-covid-19-vaccine-doesn-t-pass-smell-test-critics-say (accessed on 25 December 2020).
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Xabier, M.; Patel, N.; Tian, J.-H.; Zhou, B.; Maciejewski, S.; Lam, K.; Portnoff, A.D.; Massare, M.J.; Frieman, M.B.; Piedra, P.A.; et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine 2020, 38, 7892–7896. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Sinovac’s Covid-19 Vaccine Gets Emergency Use Approval in China. Available online: https://www.pharmaceutical-technology.com/news/sinovac-vaccine-emergency-use (accessed on 25 December 2020).
- Johnson & Johnson Prepares to Resume Phase 3 ENSEMBLE Trial of Its Janssen COVID-19 Vaccine Candidate in the U.S. Available online: https://www.jnj.com/our-company/johnson-johnson-prepares-to-resume-phase-3-ensemble-trial-of-its-janssen-covid-19-vaccine-candidate-in-the-us (accessed on 26 December 2020).
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; De Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. RNA-Based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef] [PubMed]
- U.K. Approves Pfizer’s Covid-19 Vaccine, Putting Pressure on FDA. Available online: https://www.statnews.com/2020/12/02/u-k-approves-pfizers-covid-19-vaccine-putting-pressure-on-fda/#:~:text=U.K.%20approves%20Pfizer’s%20Covid%2D19%20vaccine%2C%20putting%20pressure%20on%20FDA,-By%20Matthew%20Herper&text=The%20United%20Kingdom%20on,swiftly%20to%20do%20the%20same (accessed on 20 January 2021).
- BioNTech, Pfizer, and Fosun Pharma—BNT162b2. Available online: https://www.genengnews.com/covid-19-candidates/biontech-pfizer-and-fosun-pharma-bnt162/ (accessed on 26 November 2020).
- Mahase, E. Covid-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows. BMJ 2020, 371, m4826. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: People with history of significant allergic reactions should not receive Pfizer vaccine, says regulator. BMJ 2020, 371, m4780. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- mRNA-1273 Clinical Development Program. Available online: https://investors.modernatx.com/static-files/34f97bb2-d89a-45e4-a770-cae0591fa807 (accessed on 25 December 2020).
- Nichol, A.A. Potential Implications of Testing an Experimental mRNA-Based Vaccine during an Emerging Infectious Disease Pandemic. Am. J. Bioeth. 2020, 20, W2–W3. [Google Scholar] [CrossRef] [PubMed]
- Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization. Available online: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study (accessed on 25 December 2020).
- Hellfritzsch, M.; Scherließ, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandtzaeg, P. Potential of Nasopharynx-associated Lymphoid Tissue for Vaccine Responses in the Airways. Am. J. Respir. Crit. Care Med. 2011, 183, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Digestive System Is a Potential Route of COVID-19: An Analysis of Single-Cell Coexpression Pattern of Key Proteins in Viral Entry Process. Available online: https://0-gut-bmj-com.brum.beds.ac.uk/content/69/6/1010 (accessed on 19 November 2020).
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef]
- Ramirez, J.E.V.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, A.; Mathena, J.; Albano, J.D.; Yacovone, M.; Collins, L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine 2016, 34, 4558–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaxart’s Oral COVID-19 Tablet Vaccine to Enter Clinical Trials. Available online: https://www.biopharma-reporter.com/Article/2020/09/15/Vaxart-First-tablet-COVID-19-vaccine-to-enter-clinical-trials (accessed on 19 November 2020).
- Pre-Clinical Studies of a Recombinant Adenoviral Mucosal Vaccine to Prevent SARS-CoV-2 Infection. Available online: https://www.biorxiv.org/content/10.1101/2020.09.04.283853v1 (accessed on 23 December 2020).
- Chumakov, K.; Benn, C.S.; Aaby, P.; Kottilil, S.; Gallo, R. Can existing live vaccines prevent COVID-19? Science 2020, 368, 1187–1188. [Google Scholar] [CrossRef]
- Vaxart Has a Development Program Focused on Prophylactic and Therapeutic Vaccines in Multiple Indications. Available online: https://vaxart.com/pipeline/ (accessed on 19 January 2021).
- Stabilitech Biopharma Announces Name Change to iosBio. Available online: https://www.globenewswire.com/news-release/2020/09/30/2101099/0/en/Stabilitech-Biopharma-announces-name-change-to-iosBio.html (accessed on 25 December 2020).
- ORAPRO-COVID-19 Vaccine Capsules—Thermally Stable and Orally Administered. Available online: https://www.stabilitech.com/orapro-covid-19 (accessed on 19 November 2020).
- Grassly, N.C.; Jafari, H.; Bahl, S.; Sethi, R.; Deshpande, J.M.; Wolff, C.; Sutter, R.W.; Aylward, R.B. Waning Intestinal Immunity After Vaccination With Oral Poliovirus Vaccines in India. J. Infect. Dis. 2012, 205, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-S.; Lee, J.; Jung, Y.; Kang, M.-H.; Hong, J.-H.; Cha, M.-S.; Park, Y.-J.; Lee, E.; Yoon, C.-H.; Bae, Y.-S. Development of oral CTL vaccine using a CTP-integrated Sabin 1 poliovirus-based vector system. Vaccine 2015, 33, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio vaccination: Past, present and future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomoto, A.; Omata, T.; Toyoda, H.; Kuge, S.; Horie, H.; Kataoka, Y.; Genba, Y.; Nakano, Y.; Imura, N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc. Natl. Acad. Sci. USA 1982, 79, 5793–5797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Daniell, H. Long-term evaluation of mucosal and systemic immunity and protection conferred by different polio booster vaccines. Vaccine 2017, 35, 5418–5425. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, H.; Sutter, R.W.; Czerkinsky, C.; Ogra, P.L. Mucosal immunity and poliovirus vaccines: Impact on wild poliovirus infection and transmission. Vaccine 2011, 29, 8205–8214. [Google Scholar] [CrossRef] [PubMed]



| Company/ Organization | Brand Name | Vaccine Type/Platform | Phase | Country | Reference/Trial Identifier |
| Inactivated/Killed Vaccines | |||||
| Bharat Biotech, Indian Council of Medical Research, National Institute of Virology | Covaxin | Inactivated SARS-CoV-2 (multiple viral antigens) | I/II | India | NCT04471519 |
| Chinese Academy of Medical Sciences | COVID-19 vaccine | Inactivated SARS-CoV-2 (multiple viral antigens) | I/II | China | NCT04470609, NCT04470609, NCT04412538 |
| Sinovac Biotech | CoronaVac (PiCoVacc) | Inactivated SARS-CoV-2 (multiple viral antigens) | I/II/III | China, Brazil | [37] NCT04456595, NCT04383574, NCT04352608 |
| Sinopharm, Beijing Institute of Biological Products Co. Ltd | BBIBP-CorV | Inactivated SARS-CoV-2 (multiple viral antigens) | I/II/III | China | [38] ChiCTR2000030906 |
| Live attenuated Vaccines | |||||
| - | - | - | - | - | - |
| Recombinant Vaccines | |||||
| CanSino Biologics Inc., Beijing Institute of Biotechnology | Ad5-nCoV | Non-replicating adenoviral (Ad5) vector | I/II/III | China, Canada, Russia | [117,118,119] NCT04313127 NCT04313127 NCT04341389 |
| AstraZeneca, University of Oxford, Serum Institute of India | ChAdOxnCoV-19 (AZD1222) | Non-replicating viral vector (ChAdOx1)- expressing S protein | I/II | UK, South Africa USA, Brazil | [120,121] |
| Gameleya Research Institute | Sputnik V (Gam-COVID-VacLyo) | Recombinant non-replicating viral (Ad5- or Ad26)-vectored | I/II | Russia | [122] NCT04436471, NCT04437875 |
| Johnson & Johnson | Ad26.COV2-S (JNJ-78436735) | Ad26-vectored, non-replicating, nanoparticle | I/II | USA, Belgium | [123] NCT04436276 |
| Merck, IAVI | COVID-19 vaccine | VSV-vectored, replicating | I/II | USA, Austria, Belgium | NCT04498247, NCT04498247 |
| Novavax | NVX-CoV2373 | Recombinant S-protein | I/II | Australia | NCT04368988 |
| DNA-based Vaccines | |||||
| AnGes Inc., Osaka University, Takara Bio | AG0302-COVID19 | Plasmid DNA (expressing S protein) | I/II | Japan | NCT04527081, NCT04527081 |
| Entos Pharmaceuticals | Covigenix VAX-001 | Plasmid DNA (expressing S protein) | I/II | Canada, USA | NCT04591184 |
| Genexine Consortium | GX-19 | Plasmid DNA (expressing S protein) | I/II | South Korea | NCT04445389 |
| Inovio Pharmaceuticals, International Vaccine Institute | NO-4800a | Plasmid DNA (expressing S protein) | I/II/III | USA | [124] NCT04336410, NCT04447781 |
| Zydus Cadila | ZyCov-D | Plasmid DNA (expressing S protein) | I/II | India | CTRI/2020/07/026352, CTRI/2020/07/026352 |
| RNA-based Vaccines | |||||
| Academy of Military Medical Sciences, Walvax Biotechnology, Suzhou Abogen Biosciences | ARCoV | mRNA (expressing S protein) | I | China | [125] ChiCTR2000034112 |
| Arcturus Therapeutics, Duke-National University of Singapore | Lunar-COV19 | Self-replicating mRNA (expressing S protein) | I/II | Singapore | NCT04480957 NCT04480957 NCT04480957 |
| CureVac | CVnCoV | Lipid nanoparticle- mRNA | I | Germany, Belgium | NCT04449276 |
| Imperial College London, Morningside Ventures | LNP-nCoVsa- RNA | Lipid nanoparticle- saRNA (expressing S protein) | I/II | UK | [126,127] ISRCTN17072692 |
| Moderna, NIAID (VRC) | mRNA-1273 | mRNA-based (Lipid nanoparticle– mRNA) | III | USA | [97,108] NCT04283461, NCT04470427, NCT04405076 |
| Pfizer, BioNTech, Fosun Pharma | BNT162b1, BNT162b2 | mRNA-based (RBD of S-protein) | I/II/III | Germany, USA, China | NCT04368728 |
| Subunit Vaccines | |||||
| Anhui Zhifei Longcom Biologic Pharmacy, Chinese Academy of Medical Sciences | COVID-19 vaccine | Protein subunit (dimeric RBD) | I/II/III | China | NCT04445194 NCT04466085 NCT04646590 |
| Clover Pharmaceuticals, GlaxoSmithKline, Dynavax | SCB-2019 | Protein subunit (trimeric S protein) | I | Australia | [128] NCT04405908 |
| Kentucky Bioprocessing Inc. | KBPCOVID-19 | Protein subunit (RBD-protein) | I/II | USA | NCT04473690 |
| Medicago, Laval University | COVID-19 vaccine | Virus-like particle (VLP) | I | Canada | [92] NCT04450004 |
| Medigen Vaccine Biologics, Dynavax | MVC-COV1901 | Protein subunit (S-protein) | I | Taiwan | NCT04487210 |
| University of Queensland | COVID-19 vaccine | Protein subunit (molecular Clamp-stabilized S-protein) | I | Australia | NCT04495933 |
| Vaxine Pty Ltd, Medytox, Central Adelaide Local Health Network | COVAX19 | Protein subunit (S-protein with Advax-SM adjuvant) | I | Australia | NCT04453852 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.U.; Kim, Y.; Kumar, S.; Seo, D.; Ashraf, M.; Bae, Y.-S. COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform. Vaccines 2021, 9, 171. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9020171
Ashraf MU, Kim Y, Kumar S, Seo D, Ashraf M, Bae Y-S. COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform. Vaccines. 2021; 9(2):171. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9020171
Chicago/Turabian StyleAshraf, Muhammad Umer, Yeji Kim, Sunil Kumar, Dongyeob Seo, Maryam Ashraf, and Yong-Soo Bae. 2021. "COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform" Vaccines 9, no. 2: 171. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9020171