Incidence of Carassius auratus Gibelio Gill Hemorrhagic Disease Caused by CyHV-2 Infection Can Be Reduced by Vaccination with Polyhedra Incorporating Antigens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CyHV-2
2.2. Synthesis of H1-D4ORF and D4ORF-VP3 Fused DNA Sequences
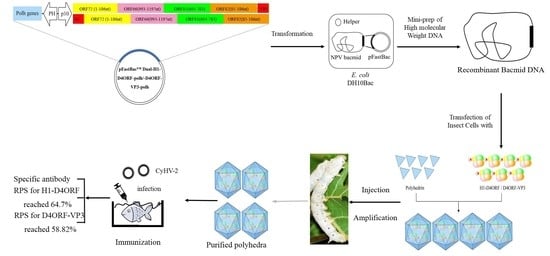
2.3. Construction of the Baculovirus Transfer Vector and Recombinant Baculovirus
2.4. Expression of H1-D4ORF and D4ORF-VP3 in the Silkworm
2.5. Purification of Polyhedra (Polyhedral Microcrystals)
2.6. Detection of H1-D4ORF and D4ORF-VP3 Recombinant Proteins in Polyhedra and Hemolymph
2.7. Storage Stability of the Recombinant Protein Incorporated into the Polyhedral Microcrystals
2.8. Vaccination
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Challenge Trials
2.11. Detection of the Incorporated Baculovirus BmNPV into BmCPV Polyhedra
3. Results
3.1. Generation of Recombinant Baculovirus and Expression of Recombinant Proteins
3.2. H1-D4ORF and D4ORF-VP3 Recombinant Proteins Can Be Incorporated into Polyhedral Microcrystals
3.3. Polyhedra Protects the Incorporated Proteins from Degradation
3.4. Injection Immunization with Polyhedron-Encapsulated H1-D4ORF/D4ORF-VP3 Fused Proteins Confers Protection against CyHV-2
3.5. Baculovirus BmNPV Can Be Incorporated into BmCPV Polyhedra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Su, H.; Su, J. Cyprinid viral diseases and vaccine development. Fish Shellfish Immunol. 2018, 83, 84–95. [Google Scholar] [CrossRef]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [Green Version]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Lorenzen, N.; Collet, B. DNA vaccination for finfish aquaculture. Fish Shellfish Immunol. 2019, 85, 106–125. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.N.; Saevareid, K.; McAuley, L.; Lane, M.E.; Bogwald, J.; Dalmo, R.A. Early immune responses in Atlantic salmon (Salmo salar L.) after immunization with PLGA nanoparticles loaded with a model antigen and beta-glucan. Vaccine 2011, 29, 8338–8349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Torrealba, D.; Ruyra, A.; Roher, N. Nanodelivery Systems as New Tools for Immunostimulant or Vaccine Administration: Targeting the Fish Immune System. Biology 2015, 4, 664–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munang’andu, H.M.; Fredriksen, B.N.; Mutoloki, S.; Brudeseth, B.; Kuo, T.Y.; Marjara, I.S.; Dalmo, R.A.; Evensen, O. Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine 2012, 30, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, X.; Wang, K.; He, J.; Zhu, L.; He, Y.; Chen, D.; Ouyang, P.; Geng, Y.; Huang, X.; et al. Preparation, characterization and evaluation of the immune effect of alginate/chitosan composite microspheres encapsulating recombinant protein of Streptococcus iniae designed for fish oral vaccination. Fish Shellfish Immunol. 2018, 73, 262–271. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Remminghorst, U.; Rehm, B.H. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef]
- Cao, G.; Meng, X.; Xue, R.; Zhu, Y.; Zhang, X.; Pan, Z.; Zheng, X.; Gong, C. Characterization of the complete genome segments from BmCPV-SZ, a novel Bombyx mori cypovirus 1 isolate. Can. J. Microbiol. 2012, 58, 872–883. [Google Scholar] [CrossRef]
- Rohrmann, G.F. Polyhedrin structure. J. Gen Virol. 1986, 67, 1499–1513. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Nakazawa, H.; Shimo-Oka, A.; Ishio, K.; Miyata, S.; Hosokawa, Y.; Matsumura, S.; Masuhara, H.; Belloncik, S.; Alain, R.; et al. Immobilization of diverse foreign proteins in viral polyhedra and potential application for protein microarrays. Proteomics 2006, 6, 54–66. [Google Scholar] [CrossRef]
- Ijiri, H.; Coulibaly, F.; Nishimura, G.; Nakai, D.; Chiu, E.; Takenaka, C.; Ikeda, K.; Nakazawa, H.; Hamada, N.; Kotani, E.; et al. Structure-based targeting of bioactive proteins into cypoviruspolyhedra and application to immobilized cytokines for mammalian cell culture. Biomaterials 2009, 30, 4297–4308. [Google Scholar] [CrossRef]
- Mori, H.; Oda, N.; Abe, S.; Ueno, T.; Zhu, W.; Pernstich, C.; Pezzotti, G. Raman spectroscopy insight into Norovirus encapsulation in Bombyx mori cypovirus cubic microcrystals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, G.; Hirohata, R.; Hayashi, K.; Sugimoto, Y.; Kotani, E.; Shimabukuro, J.; Hirano, T.; Nakajima, Y.; Kawamata, S.; Mori, H. Control of angiogenesis by VEGF and endostatin-encapsulated protein microcrystals and inhibition of tumor angiogenesis. Biomaterials 2014, 35, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Ijiri, H.; Negishi, H.; Yamanaka, H.; Sasaki, K.; Hirata, K.; Mori, H.; Ueno, T. Design of Enzyme-Encapsulated Protein Containers by In Vivo Crystal Engineering. Adv. Mater. 2015, 27, 7951–7956. [Google Scholar] [CrossRef]
- Mori, H.; Shukunami, C.; Furuyama, A.; Notsu, H.; Nishizaki, Y.; Hiraki, Y. Immobilization of bioactive fibroblast growth factor-2 into cubic proteinous microcrystals (Bombyx mori cypoviruspolyhedra) that are insoluble in a physiological cellular environment. J. Biol. Chem. 2007, 282, 17289–17296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimabukuro, J.; Yamaoka, A.; Murata, K.; Kotani, E.; Hirano, T.; Nakajima, Y.; Matsumoto, G.; Mori, H. 3D co-cultures of keratinocytes and melanocytes and cytoprotective effects on keratinocytes against reactive oxygen species by insect virus-derived protein microcrystals. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Nishishita, N.; Ijiri, H.; Takenaka, C.; Kobayashi, K.; Goto, K.; Kotani, E.; Itoh, T.; Mori, H.; Kawamata, S. The use of leukemia inhibitory factor immobilized on virus-derived polyhedra to support the proliferation of mouse embryonic and induced pluripotent stem cells. Biomaterials 2011, 32, 3555–3563. [Google Scholar] [CrossRef]
- Matsumoto, G.; Ueda, T.; Shimoyama, J.; Ijiri, H.; Omi, Y.; Yube, H.; Sugita, Y.; Kubo, K.; Maeda, H.; Kinoshita, Y. Bone regeneration by polyhedral microcrystals from silkworm virus. Sci. Rep. 2012, 2, 935. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Liang, L.; Zheng, X.; Jia, P.; Shi, X.; Lan, W.; Xie, J.; Lui, H.; Xu, P. Mass mortality caused by Cyprinid Herpesvirus 2 (CyHV-2) in Prussian carp (Carassiusgibelio) in China. Bull. Eur. Ass. Fish Pathol. 2012, 32, 164–173. [Google Scholar]
- Wu, T.; Ding, Z.; Ren, M.; An, L.; Xiao, Z.; Liu, P.; Gu, W.; Meng, Q.; Wang, W. The histo-and ultra-pathological studies on a fatal disease of Prussian carp (Carassiusgibelio) in mainland China associated with cyprinid herpesvirus 2 (CyHV-2). J. Aquac. 2013, 412–413, 8–13. [Google Scholar] [CrossRef]
- Ito, T.; Maeno, Y. Effect of booster shot and investigation of vaccination efficacy period against herpesviralhaematopoietic necrosis (HVHN) in goldfish Carassius auratus. Vet. Microbiol. 2015, 175, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Fan, Y.; Zhou, Y.; Xu, J.; Liu, W.; Gu, Z.; Zeng, L. Immune response and protection in gibel carp, Carassiusgibelio, after vaccination with beta-propiolactone inactivated cyprinid herpesvirus 2. Fish Shellfish Immunol. 2016, 49, 344–350. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, N.; Ma, J.; Fan, Y.; Zhang, L.; Xu, J.; Zeng, L. Protective immunity in gibel carp, Carassiusgibelio of the truncated proteins of cyprinid herpesvirus 2 expressed in Pichia pastoris. Fish Shellfish Immunol. 2015, 47, 1024–1031. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, S.; Nan, H.; Zhao, K.; Xu, X.; Wang, G.; Ji, H.; Chen, H. Immersion immunization with recombinant baculoviruses displaying cyprinid herpesvirus 2 membrane proteins induced protective immunity in gibel carp. Fish Shellfish Immunol. 2019, 93, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yuan, R.; Zhang, M.; Zhang, T.; Gu, Y.; Zhou, Y.; Dai, Y.; Fang, P.; Feng, Y.; Hu, X.; et al. Recombinant baculovirus BacCarassius-D4ORFs has potential as a live vector vaccine against CyHV-2. Fish Shellfish Immunol. 2019, 92, 101–110. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, B.; Cao, G.; Hu, X.; Wei, Y.; Yi, J.; Zhou, Y.; Pan, G.; Wang, J.; Xue, R.; et al. Identification and rapid diagnosis of the pathogen responsible for haemorrhagic disease of the gill of Allogynogenetic crucian carp. J. Virol. Methods 2015, 219, 67–74. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xue, R.Y.; Cao, G.L.; Meng, X.K.; Zhu, Y.X.; Pan, Z.H.; Gong, C.L. Nonvirus encoded proteins could be embedded into Bombyx mori cypoviruspolyhedra. Mol. Biol. Rep. 2014, 41, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, L.; Cao, G.; Xu, S.; Li, J.; Zou, Y.; Chen, H.; Gong, C. Oral vaccination of BacFish-vp6 against grass carp reovirus evoking antibody response in grass carp. Fish Shellfish Immunol. 2013, 34, 348–355. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, G.; Zhu, L.; Chen, F.; Zar, M.S.; Wang, S.; Hu, X.; Wei, Y.; Xue, R.; Gong, C. Integrin beta and receptor for activated protein kinase C are involved in the cell entry of Bombyx mori cypovirus. Appl. Microbiol. Biotechnol. 2017, 101, 3703–3716. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Fan, C.; Ai, T.; Su, J. The combination of molecular adjuvant CCL35.2 and DNA vaccine significantly enhances the immune protection ofCarassius auratus gibelioagainst CyHV-2 Infection. Vaccines 2020, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.L.; Chen, T.J.; Huang, Y.; Zeng, W.W.; Xin-Bing, Y.U.; Parasitology, D.O. Journal of Tropical Medicine Prokaryotic expression and immunization analysis of grass carp reovirus HZ08 strain outer capsid protein VP4 segment. J. Trop. Med. 2016, 16, 140–144. [Google Scholar]
- Cui, L.C.; Guan, X.T.; Liu, Z.M.; Tian, C.Y.; Xu, Y.G. Recombinant lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): A promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination. Vaccine 2015, 33, 3092–3099. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Rigaudeau, D.; Tacchi, L.; Pijlman, G.P.; Kampers, L.; Vesely, T.; Pokorova, D.; Boudinot, P.; Wiegertjes, G.F.; Forlenza, M. Vaccination of carp against SVCV with an oral DNA vaccine or an insect cells-based subunit vaccine. Fish Shellfish Immunol. 2019, 85, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.; Chiu, E.; Ikeda, K.; Gutmann, S.; Haebel, P.W.; Schulze-Briese, C.; Mori, H.; Metcalf, P. The molecular organization of cypoviruspolyhedra. Nature 2007, 446, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus induces an innate immune response and confers protectionfrom lethal influenza virus infection in mice. J. Immunol. 2003, 171, 1133–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, N.B.; Sidhu, J.S.; Omiecinski, C.J. Baculovirus vectors repress phenobarbitalmediatedgene induction and stimulate cytokine expression in primarycultures of rat hepatocytes. Gene Ther. 2000, 7, 1274–1283. [Google Scholar] [CrossRef] [PubMed]





| Group | Control | H1-D4ORF | D4ORF-VP3 |
|---|---|---|---|
| Injection Dose | PBS | 7 × 106 per fish | 4.4 × 106 per fish |
| Infection Rate (%) | 17/20 | 6/20 | 7/20 |
| Relative Percent Survival (%) | / | 64.7 | 58.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Gu, Y.; Liu, X.; Yuan, R.; Zhou, Y.; Dai, Y.; Fang, P.; Feng, Y.; Cao, G.; Chen, H.; et al. Incidence of Carassius auratus Gibelio Gill Hemorrhagic Disease Caused by CyHV-2 Infection Can Be Reduced by Vaccination with Polyhedra Incorporating Antigens. Vaccines 2021, 9, 397. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9040397
Zhang T, Gu Y, Liu X, Yuan R, Zhou Y, Dai Y, Fang P, Feng Y, Cao G, Chen H, et al. Incidence of Carassius auratus Gibelio Gill Hemorrhagic Disease Caused by CyHV-2 Infection Can Be Reduced by Vaccination with Polyhedra Incorporating Antigens. Vaccines. 2021; 9(4):397. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9040397
Chicago/Turabian StyleZhang, Tingting, Yuchao Gu, Xiaohan Liu, Rui Yuan, Yang Zhou, Yaping Dai, Ping Fang, Yongjie Feng, Guangli Cao, Hui Chen, and et al. 2021. "Incidence of Carassius auratus Gibelio Gill Hemorrhagic Disease Caused by CyHV-2 Infection Can Be Reduced by Vaccination with Polyhedra Incorporating Antigens" Vaccines 9, no. 4: 397. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9040397