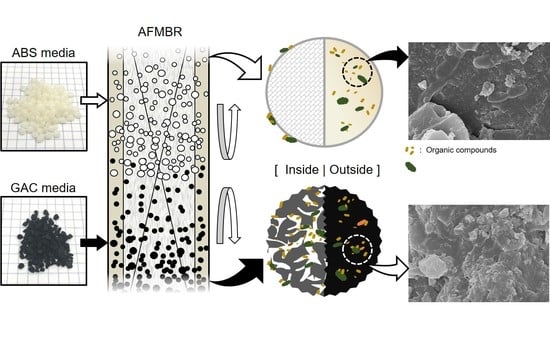
Combined Effect of Activated Carbon Particles and Non-Adsorptive Spherical Beads as Fluidized Media on Fouling, Organic Removal and Microbial Communities in Anaerobic Membrane Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. AFMBR Operation
2.2. Selection of Fluidized Media
2.3. Operation of AFMBR
2.4. Microorganism Analysis
2.5. Analytical Methods
3. Results and Discussion
3.1. Effect of Fluidization of Combined Media on Membrane Fouling
3.2. AFMBR Treatment Efficiency
3.3. Microbial Analysis
3.4. Energy Requirements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Kim, K.; Ye, H.; Lee, E.; Shin, C.; McCarty, P.L.; Bae, J. Anaerobic Fluidized Bed Membrane Bioreactor for Wastewater Treatment. Environ. Sci. Technol. 2011, 45, 576–581. [Google Scholar] [CrossRef]
- Gao, D.-W.; Hu, Q.; Yao, C.; Ren, N.-Q.; Wu, W.-M. Integrated anaerobic fluidized-bed membrane bioreactor for domestic wastewater treatment. Chem. Eng. J. 2014, 240, 362–368. [Google Scholar] [CrossRef]
- Wu, B.; Wong, P.C.Y.; Fane, A.G. The potential roles of granular activated carbon in anaerobic fluidized membrane bioreactors: Effect on membrane fouling and membrane integrity. Desalination Water Treat. 2015, 53, 1450–1459. [Google Scholar] [CrossRef]
- Lee, E.; Rout, P.R.; Shin, C.; Bae, J. Effects of sodium hypochlorite concentration on the methanogenic activity in an anaerobic fluidized membrane bioreactor. Sci. Total Environ. 2019, 678, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Düppenbecker, B.; Engelhart, M.; Cornel, P. Fouling mitigation in Anaerobic Membrane Bioreactor using fluidized glass beads: Evaluation fitness for purpose of ceramic membranes. J. Membr. Sci. 2017, 537, 69–82. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Abu Hassan, M.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Huang, L.; Lee, D.J. Membrane bioreactor: A mini review on recent R&D works. Bioresour. Technol. 2015, 194, 383–388. [Google Scholar] [CrossRef]
- Mustafa, N.; Elbeshbishy, E.; Nakhla, G.; Zhu, J. Anaerobic digestion of municipal wastewater sludges using anaerobic fluidized bed bioreactor. Bioresour. Technol. 2014, 172, 461–466. [Google Scholar] [CrossRef]
- Akram, A.; Stuckey, D.C. Flux and performance improvement in a submerged anaerobic membrane bioreactor (SAMBR) using powdered activated carbon (PAC). Process Biochem. 2008, 43, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Damayanti, A.; Ujang, Z.; Salim, M.R. The influenced of PAC, zeolite, and Moringa oleifera as biofouling reducer (BFR) on hybrid membrane bioreactor of palm oil mill effluent (POME). Bioresour. Technol. 2011, 102, 4341–4346. [Google Scholar] [CrossRef]
- Aun Ng, C.; Sun, D.; Fane, A.G. Operation of Membrane Bioreactor with Powdered Activated Carbon Addition. Sep. Sci. Technol. 2006, 41, 1447–1466. [Google Scholar] [CrossRef]
- Chen, W.-H.; Tsai, C.-Y.; Chen, S.-Y.; Sung, S.; Lin, J.-G. Treatment of campus domestic wastewater using ambient-temperature anaerobic fluidized membrane bioreactors with zeolites as carriers. Int. Biodeterior. Biodegrad. 2019, 136, 49–54. [Google Scholar] [CrossRef]
- Fernandez, N.; Montalvo, S.; Guerrero, L.; Sanchez, E.; Cortes, I.; Travieso, L. Anaerobic fluidized bed reactor application to tropical fruit wine effluent. Water Sci. Technol. 2007, 56, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Yuniarto, A.; Olsson, G. Membrane bioreactor: Applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 2013, 225, 109–119. [Google Scholar] [CrossRef]
- Shin, C.; McCarty, P.L.; Kim, J.; Bae, J. Pilot-scale temperate-climate treatment of domestic wastewater with a staged anaerobic fluidized membrane bioreactor (SAF-MBR). Bioresour. Technol. 2014, 159, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.; Kim, J.; McCarty, P.L.; Bae, J. Anaerobic treatment of municipal wastewater with a staged anaerobic fluidized membrane bioreactor (SAF-MBR) system. Bioresour. Technol. 2012, 120, 133–139. [Google Scholar] [CrossRef]
- Bae, J.; Shin, C.; Lee, E.; Kim, J.; McCarty, P.L. Anaerobic treatment of low-strength wastewater: A comparison between single and staged anaerobic fluidized bed membrane bioreactors. Bioresour. Technol. 2014, 165, 75–80. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Shao, S.; Cai, L.; Li, K.; Li, J.; Du, X.; Li, G.; Liang, H. Deposition of powdered activated carbon (PAC) on ultrafiltration (UF) membrane surface: Influencing factors and mechanisms. J. Membr. Sci. 2017, 530, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.A.; Sun, D.; Zhang, J.; Wu, B.; Fane, A.G. Mechanisms of Fouling Control in Membrane Bioreactors by the Addition of Powdered Activated Carbon. Sep. Sci. Technol. 2010, 45, 873–889. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.-W.; Kim, S.; Park, P.-K.; Kim, J.-H.; Lee, C.-H. Removal of 17β-estradiol by powdered activated carbon—Microfiltraion hybrid process: The effect of PAC deposition on membrane surface. J. Membr. Sci. 2009, 326, 84–91. [Google Scholar] [CrossRef]
- Aslam, M.; McCarty, P.L.; Bae, J.; Kim, J. The effect of fluidized media characteristics on membrane fouling and energy consumption in anaerobic fluidized membrane bioreactors. Sep. Purif. Technol. 2014, 132, 10–15. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef]
- Charfi, A.; Park, E.; Aslam, M.; Kim, J. Particle-sparged anaerobic membrane bioreactor with fluidized polyethylene terephthalate beads for domestic wastewater treatment: Modelling approach and fouling control. Bioresour. Technol. 2018, 258, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.E.; Chhetri, R.K.; Ooi, G.; Hansen, K.M.; Litty, K.; Christensson, M.; Kragelund, C.; Andersen, H.R.; Bester, K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res. 2015, 83, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Barwal, A.; Chaudhary, R. To study the performance of biocarriers in moving bed biofilm reactor (MBBR) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems: A review. Rev. Environ. Sci. Biotechnol. 2014, 13, 285–299. [Google Scholar] [CrossRef]
- Balart, R.; Lopez, J.; García, D.; Salvador, M.D. Recycling of ABS and PC from electrical and electronic waste. Effect of miscibility and previous degradation on final performance of industrial blends. Eur. Polym. J. 2005, 41, 2150–2160. [Google Scholar] [CrossRef]
- Yang, S.; Castilleja, J.R.; Barrera, E.; Lozano, K. Thermal analysis of an acrylonitrile-butadiene-styrene/SWNT composite. Polym. Degrad. Stab. 2004, 83, 383–388. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green Sustain. Chem. 2018, 13, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Peszynska, M.; Trykozko, A.; Iltis, G.; Schlueter, S.; Wildenschild, D. Biofilm growth in porous media: Experiments, computational modeling at the porescale, and upscaling. Adv. Water Resour. 2016, 95, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lee, C.; Kim, S.W.; Kim, C.S.; Kim, I.S. Performance Evaluation and Fouling Propensity of Forward Osmosis (FO) Membrane for Reuse of Spent Dialysate. Membranes 2020, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Pirbazari, M.; Voice, T.C.; Weber, W.J. Evaluation of Biofilm Development on Various Natural and Synthetic Media. Hazard. Waste Hazard. Mater. 1990, 7, 239–250. [Google Scholar] [CrossRef]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Desrousseaux, C.; Cueff, R.; Aumeran, C.; Garrait, G.; Mailhot-Jensen, B.; Traoré, O.; Sautou, V. Fabrication of Acrylonitrile-Butadiene-Styrene Nanostructures with Anodic Alumina Oxide Templates, Characterization and Biofilm Development Test for Staphylococcus epidermidis. PLoS ONE 2015, 10, e0135632. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.M. Effects of triclosan incorporation into ABS plastic on biofilm communities. J. Antimicrob. Chemother. 2004, 53, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Kwon, D.; Chang, H.; Seo, H.; Kim, J. Fouling behavior and system performance in membrane bioreactor introduced by granular media as a mechanical cleaning effect on membranes. Desalination Water Treat. 2016, 57, 9018–9026. [Google Scholar] [CrossRef]
- Vo, T.-K.-Q.; Lee, J.-J.; Kang, J.-S.; Park, S.; Kim, H.-S. Nitrogen Removal by Sulfur-Based Carriers in a Membrane Bioreactor (MBR). Membranes 2018, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschka, S.; Patterson, J.; Nolet, C. Machine Learning in Python: Main Developments and Technology Trends in Data Science, Machine Learning, and Artificial Intelligence. Information 2020, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, E.W.; Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association and Water Environment Federation: Washington, DC, USA, 2017; pp. 144–153. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Siembida, B.; Cornel, P.; Krause, S.; Zimmermann, B. Effect of mechanical cleaning with granular material on the permeability of submerged membranes in the MBR process. Water Res. 2010, 44, 4037–4046. [Google Scholar] [CrossRef]
- Basu, O.D.; Huck, P.M. Impact of support media in an integrated biofilter-submerged membrane system. Water Res. 2005, 39, 4220–4228. [Google Scholar] [CrossRef]
- Johir, M.A.H.; Aryal, R.; Vigneswaran, S.; Kandasamy, J.; Grasmick, A. Influence of supporting media in suspension on membrane fouling reduction in submerged membrane bioreactor (SMBR). J. Membr. Sci. 2011, 374, 121–128. [Google Scholar] [CrossRef]
- Hu, A.Y.; Stuckey, D.C. Activated carbon addition to a submerged anaerobic membrane bioreactor: Effect on performance, transmembrane pressure, and flux. J. Environ. Eng. 2007, 133, 73–80. [Google Scholar] [CrossRef]
- Dutta, K.; Lee, M.Y.; Lai, W.W.; Lee, C.H.; Lin, A.Y.; Lin, C.F.; Lin, J.G. Removal of pharmaceuticals and organic matter from municipal wastewater using two-stage anaerobic fluidized membrane bioreactor. Bioresour. Technol. 2014, 165, 42–49. [Google Scholar] [CrossRef]
- Shin, C.; Kim, K.; McCarty, P.L.; Kim, J.; Bae, J. Development and application of a procedure for evaluating the long-term integrity of membranes for the anaerobic fluidized membrane bioreactor (AFMBR). Water Sci. Technol. 2016, 74, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, B.; Yang, S.; Liu, Y.; Fane, A.G.; Chew, J.W. Characterizing the scouring efficiency of Granular Activated Carbon (GAC) particles in membrane fouling mitigation via wavelet decomposition of accelerometer signals. J. Membr. Sci. 2016, 498, 105–115. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; McCarty, P.L.; Shin, C.; Bae, J.; Kim, J. Low energy single-staged anaerobic fluidized bed ceramic membrane bioreactor (AFCMBR) for wastewater treatment. Bioresour. Technol. 2017, 240, 33–41. [Google Scholar] [CrossRef]
- Kochkodan, V.; Tsarenko, S.; Potapchenko, N.; Kosinova, V.; Goncharuk, V. Adhesion of microorganisms to polymer membranes: A photobactericidal effect of surface treatment with TiO2. Desalination 2008, 220, 380–385. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Rigby, M.G.; Argo, D.G. Bacterial adhesion and fouling of reverse osmosis membranes. J. Am. Water Work. Assoc. 1985, 77, 97–106. [Google Scholar] [CrossRef]
- Gallardo-Moreno, A.M.; González-Martín, M.L.; Bruque, J.M.; Pérez-Giraldo, C. The adhesion strength of Candida parapsilosis to glass and silicone as a function of hydrophobicity, roughness and cell morphology. Colloids Surf. Physicochem. Eng. Asp. 2004, 249, 99–103. [Google Scholar] [CrossRef]
- Kim, M.; Lam, T.Y.C.; Tan, G.-Y.A.; Lee, P.-H.; Kim, J. Use of polymeric scouring agent as fluidized media in anaerobic fluidized bed membrane bioreactor for wastewater treatment: System performance and microbial community. J. Membr. Sci. 2020, 606, 118121. [Google Scholar] [CrossRef]
- Yang, P.; Tan, G.A.; Aslam, M.; Kim, J.; Lee, P.H. Metatranscriptomic evidence for classical and RuBisCO-mediated CO2 reduction to methane facilitated by direct interspecies electron transfer in a methanogenic system. Sci Rep. 2019, 9, 4116. [Google Scholar] [CrossRef] [PubMed]
- Pagani, I.; Lapidus, A.; Nolan, M.; Lucas, S.; Hammon, N.; Deshpande, S.; Cheng, J.F.; Chertkov, O.; Davenport, K.; Tapia, R.; et al. Complete genome sequence of Desulfobulbus propionicus type strain (1pr3). Stand. Genom. Sci. 2011, 4, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovley, D.R. Syntrophy Goes Electric: Direct Interspecies Electron Transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.R.; McInerney, M.J.; Gunsalus, R.P. Genomic insights into syntrophy: The paradigm for anaerobic metabolic cooperation. Annu. Rev. Microbiol. 2012, 66, 429–452. [Google Scholar] [CrossRef] [PubMed]







| ABS Plastic Beads | GAC Particles | |
|---|---|---|
| Specific gravity | 1.04 | 2.00 |
| Diameter (mm) | 2~3 | 1~2 (>0.84) |
| Surface area (m2/g) | 0.28 | 700~1300 |
| Moisture absorption (%) | 0.95 | 2.00 |
| Shape | Flat-sphere | Angular-sphere |
| Period | 1 | 2 | 3 |
|---|---|---|---|
| Day | 0~90 | 91~110 | 111~180 |
| Flux (L/m2h) | 5.3 | 7.1 | 7.1 |
| HRT (h) | 8 | 6 | 6 |
| Relaxation | Filtration 9 min Relaxation 1 min | ||
| Recirculation rate (L/min) | 3 | 3 | 3 |
| Temperature (°C) | 25 | 25 | 25 |
| Period | 1 | 2 | 3 |
|---|---|---|---|
| Day | 0~90 | 91~110 | 111~180 |
| SCOD removal (%) | 58.5 ± 28.3 | 89.3 ± 4.4 | 95.3 ± 3.2 |
| VSS in bulk (mg/L) | 370.8 ± 100.0 | 60.0 ± 28.9 | 211.7 ± 92.5 |
| Biogas (L/d) | <1 | 1 | 1 |
| Methane (CH4, %) | 37.1 ± 27.3 | 60.1 ± 5.1 | 56.3 ± 2.5 |
| Phyla | Bulk | GAC | ABS |
|---|---|---|---|
| Acidobacteria | 0.0081 | 0.0351 | 0.0459 |
| Bacteroidetes | 0.1964 | 0.1601 | 0.0987 |
| Caldiserica | 0.0032 | 0.0187 | 0.0124 |
| Chloroflexi | 0.0851 | 0.1701 | 0.1671 |
| Firmicutes | 0.0099 | 0.0162 | 0.0423 |
| Omnitrophicaeota | 0.0053 | 0.0345 | 0.0346 |
| Patescibacteria | 0.0352 | 0.1611 | 0.0768 |
| Planctomycetes | 0.0106 | 0.0197 | 0.0685 |
| Proteobacteria | 0.5090 | 0.3071 | 0.2854 |
| Spirochaetes | 0.0082 | 0.0090 | 0.0364 |
| Synergistetes | 0.0031 | 0.0183 | 0.0180 |
| Electrical Energy Required | |
|---|---|
| - Energy for media fluidization and influent AFMBR | |
| - Reactor head loss (mH2O) | 1.00 × 10−2 |
| - Reactor influent plus recirculation flow rate (m3/s) | 5.01 × 10−5 |
| - Fluidization energy requirement (kW) | 4.92 × 10−6 |
| - Required pumping energy (kWh/m3) | 9.84 × 10−3 |
| - Energy for permeation (permeate production) | |
| - Average TMP (mH2O) | 1.23984 |
| - Permeate flowrate (m3/s) | 1.47 × 10−7 |
| - Permeate energy requirement (kW) | 1.79 × 10−6 |
| - Required pumping energy (kWh/m3) | 3.38 × 10−3 |
| - Total pumping energy (fluidization + permeation) (kWh/m3) | 1.32 × 10−2 |
| - Total electrical energy required for pumps (fluidization + permeation (kWh/m3) (65%) | 2.03 × 10−2 |
| Electrical Energy Production Potential from Methane | |
| - Methane production (mol/m3 wastewater) | 2.23 |
| - Methane energy content (kWh/m3) (0.22 kWh/mol CH4) | 0.49 |
| - Electrical energy production from methane (kWh/m3) (33%) | 1.62 × 10−1 |
| Required/produced energy | 12.55% |
| Electrical energy produced/required | 7.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Lam, T.Y.C.; Kim, M.; Tan, G.-Y.A.; Lee, P.-H.; Kim, J. Combined Effect of Activated Carbon Particles and Non-Adsorptive Spherical Beads as Fluidized Media on Fouling, Organic Removal and Microbial Communities in Anaerobic Membrane Bioreactor. Membranes 2021, 11, 365. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11050365
Kwon D, Lam TYC, Kim M, Tan G-YA, Lee P-H, Kim J. Combined Effect of Activated Carbon Particles and Non-Adsorptive Spherical Beads as Fluidized Media on Fouling, Organic Removal and Microbial Communities in Anaerobic Membrane Bioreactor. Membranes. 2021; 11(5):365. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11050365
Chicago/Turabian StyleKwon, Daeeun, Theo Y.C. Lam, Minseok Kim, Giin-Yu Amy Tan, Po-Heng Lee, and Jeonghwan Kim. 2021. "Combined Effect of Activated Carbon Particles and Non-Adsorptive Spherical Beads as Fluidized Media on Fouling, Organic Removal and Microbial Communities in Anaerobic Membrane Bioreactor" Membranes 11, no. 5: 365. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11050365