Air Pollution—An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis
Abstract
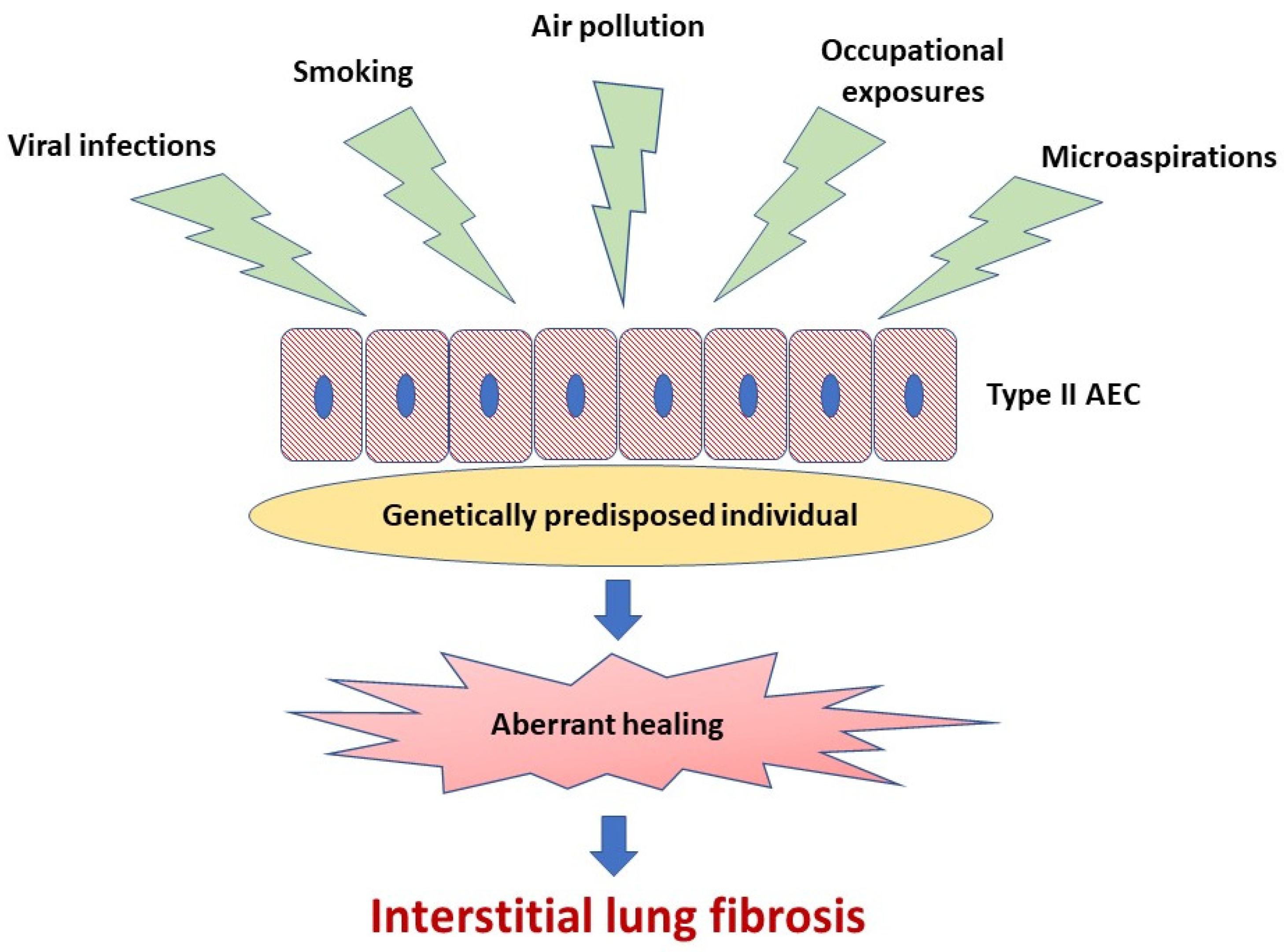
:1. Introduction
2. Types and Sources of Air Pollutants and Potential Mechanisms Triggered by Air Pollutants Affecting the Risk and Health Outcomes in IPF
2.1. Types and Sources of Air Pollutants
2.2. Potential Mechanisms Triggered by Air Pollutants Affecting the Risk and Health Outcomes in IPF
2.2.1. Oxidative Stress
2.2.2. Mitochondrial Dysfunction
2.2.3. Telomere Shortening
2.2.4. Cellular Senescence
2.2.5. Extracellular Matrix Remodeling
3. Impact of Air-Pollution on Disease Initiation and Health Outcomes in IPF
3.1. Clinical Studies Linking Air Pollution with Initiation of IPF
3.2. Clinical Studies Linking Air Pollution with the Short and Long-Term Health Outcomes in IPF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IPF | idiopathic pulmonary fibrosis |
| AE-IPF | acute exacerbation of idiopathic pulmonary fibrosis |
| FVC | forced vital capacity |
| ILAs | interstitial lung abnormalities |
| ILDs | interstitial lung diseases |
| NO2 | nitric dioxide |
| NOx | nitric oxides |
| PM2.5 | particulate matter < 2.5 μm |
| PM10 | particulate matter < 10 μm |
| O3 | ozone. |
References
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 6 November 2020).
- Yuksel, H.; Turkeli, A. Airway epithelial barrier dysfunction in the pathogenesis and prognosis of respiratory tract diseases in childhood and adulthood. Tissue Barriers 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary Health Effects of Air Pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chen, Z.; Zhou, L.-F.; Huang, S.-X. Air pollutants and early origins of respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 75–94. [Google Scholar] [CrossRef]
- Ruttens, D.; Verleden, S.E.; Bijnens, E.M.; Winckelmans, E.; Gottlieb, J.; Warnecke, G.; Meloni, F.; Morosini, M.; Van Der Bij, W.; Verschuuren, E.A.; et al. An association of particulate air pollution and traffic exposure with mortality after lung transplantation in Europe. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Maynard, R.L.; Holgate, S.T.; Koren, H.S.; Samet, J.M. (Eds.) Air Pollution and Health, 1st ed.; Academic Press: San Diego, CA, USA, 1999; ISBN 978-0-12-352335-8. [Google Scholar]
- Jacobs, E.T.; Burgess, J.L.; Abbott, M.B. The Donora Smog Revisited: 70 Years After the Event That Inspired the Clean Air Act. Am. J. Public Heal. 2018, 108, S85–S88. [Google Scholar] [CrossRef]
- Hoxha, M.; Dioni, L.; Bonzini, M.; Pesatori, A.C.; Fustinoni, S.; Cavallo, D.; Carugno, M.; Albetti, B.; Marinelli, B.; Schwartz, J.; et al. Association between leukocyte telomere shortening and exposure to traffic pollution: A cross-Sectional study on traffic officers and indoor office workers. Environ. Health Glob. Access. Sci. Source 2009, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, G.; Fu, L.; Zhong, W.; Li, X.; Pan, Q. DNA repair enzyme OGG1 promotes alveolar progenitor cell renewal and relieves PM2.5-Induced lung injury and fibrosis. Ecotoxicol. Environ. Saf. 2020, 205, 111283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Usinger, W.; Nichols, B.; Gray, J.; Xu, L.; Seeley, T.W.; Brenner, M.; Guo, G.; Zhang, W.; Oliver, N.; et al. Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease. Fibrogenesis Tissue Repair 2011, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, T.H.; Dysart, M.M.; Brown, A.C.; Douglas, A.M.; Fiore, V.F.; Russell, A.G. Synergistic effects of particulate matter and substrate stiffness on epithelial-To-Mesenchymal transition. Res. Rep. Health Eff. Inst. 2014, 182, 3–41. [Google Scholar]
- Cheresh, P.; Kim, S.-J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta 2013, 1832, 1028–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Knaapen, A.M.; Begerow, J.; Birmili, W.; Borm, P.J.A.; Schins, R.P.F. Temporal variation of hydroxyl radical generation and 8-hydroxy-2′-Deoxyguanosine formation by coarse and fine particulate matter. Occup. Environ. Med. 2003, 60, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of free radicals in the toxicity of airborne fine particulate matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, X.; Zhong, L.; Cui, Q.; Hu, X.; Li, B.; Wang, Z.; Dai, Y.; Zheng, Y.; Bin, P. The use of a 0.20 μm particulate matter filter decreases cytotoxicity in lung epithelial cells following air-Liquid interface exposure to motorcycle exhaust. Environ. Pollut. 2017, 227, 287–295. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef] [Green Version]
- Bonner, J.C. Lung fibrotic responses to particle exposure. Toxicol. Pathol. 2007, 35, 148–153. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Czermak, B.J.; Bless, N.M.; Ward, P.A. NF-kappaB activation during IgG immune complex-Induced lung injury: Requirements for TNF-Alpha and IL-1beta but not complement. Am. J. Pathol. 1998, 152, 1327–1336. [Google Scholar]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Extracellular redox state: Refining the definition of oxidative stress in aging. Rejuvenation Res. 2006, 9, 169–181. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.-S.; Li, Y.; Li, Q.-H.; Li, C.-L.; Chen, J.-M.; Weng, D.; Li, H.-P. Effects of particulate matter from straw burning on lung fibrosis in mice. Environ. Toxicol. Pharmacol. 2017, 56, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangarajan, S.; Bernard, K.; Thannickal, V.J. Mitochondrial Dysfunction in Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2017, 14, S383. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial dysfunction in lung ageing and disease. Eur. Respir. Rev. 2020, 29. [Google Scholar] [CrossRef]
- Bueno, M.; Calyeca, J.; Rojas, M.; Mora, A.L. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 2020, 33, 101509. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Li, Z.; Yue, J.; Xu, M.; Zhang, Y.; Yung, K.K.L.; Li, R. Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef]
- Leclercq, B.; Kluza, J.; Antherieu, S.; Sotty, J.; Alleman, L.Y.; Perdrix, E.; Loyens, A.; Coddeville, P.; Lo Guidice, J.-M.; Marchetti, P.; et al. Air pollution-Derived PM2.5 impairs mitochondrial function in healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells. Environ. Pollut. Barking Essex 1987 2018, 243, 1434–1449. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Panduri, V.; Surapureddi, S.; Soberanes, S.; Weitzman, S.A.; Chandel, N.; Kamp, D.W. P53 mediates amosite asbestos-Induced alveolar epithelial cell mitochondria-Reguzlated apoptosis. Am. J. Respir. Cell Mol. Biol. 2006, 34, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Soberanes, S.; Panduri, V.; Mutlu, G.M.; Ghio, A.; Bundinger, G.R.S.; Kamp, D.W. p53 mediates particulate matter-Induced alveolar epithelial cell mitochondria-Regulated apoptosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1229–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zank, D.C.; Bueno, M.; Mora, A.L.; Rojas, M. Idiopathic Pulmonary Fibrosis: Aging, Mitochondrial Dysfunction, and Cellular Bioenergetics. Front. Med. 2018, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Song, J.W.; Chu, S.G.; Mizumura, K.; Osorio, J.C.; Shi, Y.; El-Chemaly, S.; Lee, C.G.; Rosas, I.O.; Elias, J.A.; et al. Epithelial Cell Mitochondrial Dysfunction and PINK1 Are Induced by Transforming Growth Factor-Beta1 in Pulmonary Fibrosis. PLoS ONE 2015, 10, e0121246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson-Casey, J.L.; Deshane, J.S.; Ryan, A.J.; Thannickal, V.J.; Carter, A.B. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity 2016, 44, 582–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Araya, J.; Minagawa, S.; Hara, H.; Saito, N.; Kadota, T.; Sato, N.; Yoshida, M.; Tsubouchi, K.; Kurita, Y.; et al. Involvement of PARK2-Mediated Mitophagy in Idiopathic Pulmonary Fibrosis Pathogenesis. J. Immunol. 2016, 197, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, D.; Cárdenes, N.; Sellarés, J.; Bueno, M.; Corey, C.; Hanumanthu, V.S.; Peng, Y.; D’Cunha, H.; Sembrat, J.; Nouraie, M.; et al. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1164–L1173. [Google Scholar] [CrossRef]
- Ryu, C.; Sun, H.; Gulati, M.; Herazo-Maya, J.D.; Chen, Y.; Osafo-Addo, A.; Brandsdorfer, C.; Winkler, J.; Blaul, C.; Faunce, J.; et al. Extracellular Mitochondrial DNA Is Generated by Fibroblasts and Predicts Death in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 1571–1581. [Google Scholar] [CrossRef]
- Bueno, M.; Zank, D.; Buendia-Roldán, I.; Fiedler, K.; Mays, B.G.; Alvarez, D.; Sembrat, J.; Kimball, B.; Bullock, J.K.; Martin, J.L.; et al. PINK1 attenuates mtDNA release in alveolar epithelial cells and TLR9 mediated profibrotic responses. PLoS ONE 2019, 14, e0218003. [Google Scholar] [CrossRef]
- Schuliga, M.; Pechkovsky, D.V.; Read, J.; Waters, D.W.; Blokland, K.E.C.; Reid, A.T.; Hogaboam, C.M.; Khalil, N.; Burgess, J.K.; Prêle, C.M.; et al. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. J. Cell. Mol. Med. 2018, 22, 5847–5861. [Google Scholar] [CrossRef]
- Daniil, Z.; Kotsiou, O.S.; Grammatikopoulos, A.; Peletidou, S.; Gkika, H.; Malli, F.; Antoniou, K.; Vasarmidi, E.; Mamuris, Z.; Gourgoulianis, K.; et al. Detection of mitochondrial transfer RNA (mt-tRNA) gene mutations in patients with idiopathic pulmonary fibrosis and sarcoidosis. Mitochondrion 2018, 43, 43–52. [Google Scholar] [CrossRef]
- Jaeger, V.K.; Lebrecht, D.; Nicholson, A.G.; Wells, A.; Bhayani, H.; Gazdhar, A.; Tamm, M.; Venhoff, N.; Geiser, T.; Walker, U.A. Mitochondrial DNA mutations and respiratory chain dysfunction in idiopathic and connective tissue disease-related lung fibrosis. Sci. Rep. 2019, 9, 5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgili, H.; Białas, A.J.; Górski, P.; Piotrowski, W.J. Telomere Abnormalities in the Pathobiology of Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2019, 8, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armanios, M.Y.; Chen, J.J.-L.; Cogan, J.D.; Alder, J.K.; Ingersoll, R.G.; Markin, C.; Lawson, W.E.; Xie, M.; Vulto, I.; Phillips, J.A.; et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007, 356, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, W.E.; Grant, S.W.; Ambrosini, V.; Womble, K.E.; Dawson, E.P.; Lane, K.B.; Markin, C.; Renzoni, E.; Lympany, P.; Thomas, A.Q.; et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax 2004, 59, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronkhite, J.T.; Xing, C.; Raghu, G.; Chin, K.M.; Torres, F.; Rosenblatt, R.L.; Garcia, C.K. Telomere shortening in familial and sporadic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 729–737. [Google Scholar] [CrossRef]
- McCracken, J.; Baccarelli, A.; Hoxha, M.; Dioni, L.; Melly, S.; Coull, B.; Suh, H.; Vokonas, P.; Schwartz, J. Annual ambient black carbon associated with shorter telomeres in elderly men: Veterans Affairs Normative Aging Study. Environ. Health Perspect. 2010, 118, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- von Zglinicki, T.; Saretzki, G.; Döcke, W.; Lotze, C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp. Cell Res. 1995, 220, 186–193. [Google Scholar] [CrossRef]
- Martens, D.S.; Nawrot, T.S. Air Pollution Stress and the Aging Phenotype: The Telomere Connection. Curr. Environ. Health Rep. 2016, 3, 258–269. [Google Scholar] [CrossRef]
- Parimon, T.; Yao, C.; Stripp, B.R.; Noble, P.W.; Chen, P. Alveolar Epithelial Type II Cells as Drivers of Lung Fibrosis in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 2269. [Google Scholar] [CrossRef] [Green Version]
- Agudelo, C.W.; Samaha, G.; Garcia-Arcos, I. Alveolar lipids in pulmonary disease. A review. Lipids Health Dis. 2020, 19, 122. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Liu, L.; Guan, L.; Wang, T.; Zhang, L.; Wang, Y.; Cao, J.; Ding, W.; Zhang, F.; et al. DDAH1 plays dual roles in PM2.5 induced cell death in A549 cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2016, 1860, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Timblin, C.; BeruBe, K.; Gordon, T.; McKinney, W.; Driscoll, K.; Vacek, P.; Mossman, B.T. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am. J. Respir. Cell Mol. Biol. 2000, 23, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.A.; Brody, A.R. Interstitial fibrosis and growth factors. Environ. Health Perspect. 2000, 108, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Jin, H.; Ullenbruch, M.; Hu, B.; Hashimoto, N.; Moore, B.; McKenzie, A.; Lukacs, N.W.; Phan, S.H. Regulation of Found in Inflammatory Zone 1 Expression in Bleomycin-Induced Lung Fibrosis: Role of IL-4/IL-13 and Mediation via STAT-6. J. Immunol. 2004, 173, 3425–3431. [Google Scholar] [CrossRef] [Green Version]
- Churg, A.; Gilks, B.; Dai, J. Induction of fibrogenic mediators by fine and ultrafine titanium dioxide in rat tracheal explants. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L975–L982. [Google Scholar] [CrossRef] [Green Version]
- Bonner, J.C.; Rice, A.B.; Lindroos, P.M.; O’Brien, P.O.; Dreher, K.L.; Rosas, I.; Alfaro-Moreno, E.; Osornio-Vargas, A.R. Induction of the Lung Myofibroblast PDGF Receptor System by Urban Ambient Particles from Mexico City. Am. J. Respir. Cell Mol. Biol. 1998, 19, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Morales-Bárcenas, R.; Chirino, Y.I.; Sánchez-Pérez, Y.; Osornio-Vargas, Á.R.; Melendez-Zajgla, J.; Rosas, I.; García-Cuellar, C.M. Particulate matter (PM₁₀) induces metalloprotease activity and invasion in airway epithelial cells. Toxicol. Lett. 2015, 237, 167–173. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Liao, Z.; Gao, S.; Hua, L.; Ye, X.; Wang, Y.; Jiang, S.; Wang, N.; Zhou, D.; et al. PM2.5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicol. Environ. Saf. 2019, 171, 112–121. [Google Scholar] [CrossRef]
- Baumgartner, K.B.; Samet, J.M.; Coultas, D.B.; Stidley, C.A.; Hunt, W.C.; Colby, T.V.; Waldron, J.A. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: A multicenter case-Control study. Collaborating Centers. Am. J. Epidemiol. 2000, 152, 307–315. [Google Scholar] [CrossRef]
- Bédard Méthot, D.; Leblanc, É.; Lacasse, Y. Meta-Analysis of Gastroesophageal Reflux Disease and Idiopathic Pulmonary Fibrosis. Chest 2019, 155, 33–43. [Google Scholar] [CrossRef]
- Pulkkinen, V.; Salmenkivi, K.; Kinnula, V.L.; Sutinen, E.; Halme, M.; Hodgson, U.; Lehto, J.; Jääskeläinen, A.; Piiparinen, H.; Kere, J.; et al. A novel screening method detects herpesviral DNA in the idiopathic pulmonary fibrosis lung. Ann. Med. 2012, 44, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Trethewey, S.P.; Walters, G.I. The Role of Occupational and Environmental Exposures in the Pathogenesis of Idiopathic Pulmonary Fibrosis: A Narrative Literature Review. Med. Kaunas. Lith. 2018, 54, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, H.; Ichinose, S.; Hosoya, T.; Ando, T.; Ikushima, S.; Oritsu, M.; Takemura, T. Inhalation of inorganic particles as a risk factor for idiopathic pulmonary fibrosis--Elemental microanalysis of pulmonary lymph nodes obtained at autopsy cases. Pathol. Res. Pract. 2007, 203, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Harari, S.; Raghu, G.; Caminati, A.; Cruciani, M.; Franchini, M.; Mannucci, P. Fibrotic interstitial lung diseases and air pollution: A systematic literature review. Eur. Respir. Rev. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Harari, S.; Caminati, A.; Zanobetti, A.; Schwartz, J.D.; Bertazzi, P.A.; Cesana, G.; Madotto, F. The association between air pollution and the incidence of idiopathic pulmonary fibrosis in Northern Italy. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [Green Version]
- Faustini, A.; Rapp, R.; Forastiere, F. Nitrogen dioxide and mortality: Review and meta-analysis of long-Term studies. Eur. Respir. J. 2014, 44, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Shull, J.G.; Pay, M.T.; Lara Compte, C.; Olid, M.; Bermudo, G.; Portillo, K.; Sellarés, J.; Balcells, E.; Vicens-Zygmunt, V.; Planas-Cerezales, L.; et al. Mapping IPF helps identify geographic regions at higher risk for disease development and potential triggers. Respirol. Carlton Vic. 2020. [Google Scholar] [CrossRef]
- Sack, C.; Vedal, S.; Sheppard, L.; Raghu, G.; Barr, R.G.; Podolanczuk, A.; Doney, B.; Hoffman, E.A.; Gassett, A.; Hinckley-Stukovsky, K.; et al. Air pollution and subclinical interstitial lung disease: The Multi-Ethnic Study of Atherosclerosis (MESA) air-Lung study. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [Green Version]
- Wells, A.U.; Kokosi, M.A. Subclinical Interstitial Lung Abnormalities: Toward the Early Detection of Idiopathic Pulmonary Fibrosis? Am. J. Respir. Crit. Care Med. 2016, 194, 1445–1446. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.B.; Li, W.; Schwartz, J.; Di, Q.; Kloog, I.; Koutrakis, P.; Gold, D.R.; Hallowell, R.W.; Zhang, C.; O’Connor, G.; et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: The Framingham Heart Study. Thorax 2019, 74, 1063–1069. [Google Scholar] [CrossRef]
- Johannson, K.A.; Vittinghoff, E.; Morisset, J.; Wolters, P.J.; Noth, E.M.; Balmes, J.R.; Collard, H.R. Air Pollution Exposure Is Associated With Lower Lung Function, but Not Changes in Lung Function, in Patients With Idiopathic Pulmonary Fibrosis. Chest 2018, 154, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Winterbottom, C.J.; Shah, R.J.; Patterson, K.C.; Kreider, M.E.; Panettieri, R.A.; Rivera-Lebron, B.; Miller, W.T.; Litzky, L.A.; Penning, T.M.; Heinlen, K.; et al. Exposure to Ambient Particulate Matter Is Associated With Accelerated Functional Decline in Idiopathic Pulmonary Fibrosis. Chest 2018, 153, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.; Blanco-Vidal, C.; Cakmak, S. The Association between Air Pollution and Hospitalization of Patients with Idiopathic Pulmonary Fibrosis in Chile: A Daily Time Series Analysis. Chest 2020, 158, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Johannson, K.A.; Vittinghoff, E.; Lee, K.; Balmes, J.R.; Ji, W.; Kaplan, G.G.; Kim, D.S.; Collard, H.R. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur. Respir. J. 2014, 43, 1124–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, C.; Raghu, G. Idiopathic pulmonary fibrosis: Unmasking cryptogenic environmental factors. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Liu, Y.; Fan, L.; Zeng, Y.; Han, H.; Zhang, H.; Yu, X.; Zhang, Y.; Huang, D.; et al. GPRC5A reduction contributes to pollutant benzo[a]pyrene injury via aggravating murine fibrosis, leading to poor prognosis of IIP patients. Sci. Total. Environ. 2020, 739, 139923. [Google Scholar] [CrossRef]
- Sesé, L.; Nunes, H.; Cottin, V.; Sanyal, S.; Didier, M.; Carton, Z.; Israel-Biet, D.; Crestani, B.; Cadranel, J.; Wallaert, B.; et al. Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax 2018, 73, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.-Y.; Kim, S.-Y.; Kim, O.-J.; Song, J.W. Nitrogen Dioxide Increases the Risk of Mortality in Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2020. [Google Scholar] [CrossRef]

| First Author, Publication Year [Reference] | Study Design | Aims of Study | Study Population | Study Findings |
|---|---|---|---|---|
| Conti S, 2018 [67] | A longitudinal retrospective cohort study | Association between long-term air pollution exposures and IPF incidence | 2090 incident IPF cases in Lombardy, Italy | An increment of 10 µg/m3 in NO2 concentration was associated with a 7.93% increase in the incidence rate of IPF. Traffic-related pollution could be involved in the initiation of the fibrotic process in IPF. |
| Shull JG, 2020 [69] | A retrospective cohort study | Cross-analysis of geographic regions of IPF cases and mapping of PM2.5 concentration | 379 patients with IPF from the registry in the Catalan region, Spain | The prevalence of IPF was higher in areas of elevated PM2.5 concentration. Certain areas with elevated air pollutants may be deserving greater analysis for screening of IPF. |
| Sack C, 2017 [70] | A prospective cohort study | Association between ambient air pollution and ILAs | 2671 participants of the Multi-Ethnic Study on Atherosclerosis (MESA), USA | Long-term exposure to ambient NOx was associated with higher prevalence of ILAs. The odds of ILAs increased 1.77-fold per 40 ppb increment in NOx. Air pollution exposures were associated with subclinical ILDs. |
| Rice MB, 2019 [72] | A longitudinal prospective cohort study | Association between long-term exposure to traffic and ambient pollutants and incidence of ILAs and their progression | 2618 Framingham study participants, USA | Higher 5-year average exposure to elemental carbon (a traffic-related PM2.5 component) was associated with 1.27 times greater odds of ILAs, and 1.33 times greater odds of ILAs progression on sequential imaging. Long-term exposure to traffic-related pollution may lead to interstitial remodelling. |
| Johansson KA, 2018 [73] | A longitudinal prospective cohort study | Association between air pollution exposure and lung function in IPF | 25 patients with IPF, USA | Increased average exposures to NO2, PM2.5 and PM10 were associated with lower FVC in patients with IPF. Air pollution may have an influence on disease severity in IPF. |
| Winterbottom CJ, 2018 [74] | A retrospective cohort study | Association between exposures to PM2.5 and PM10 and lung function decline in IPF | 135 patients with IPF, USA | A significant association between PM10 levels and the rate of decline in FVC during the study period was noted, with each mg/m3 increase in PM10 corresponding with an additional 46 mL decline in FVC yearly. Air pollution exposure was associated with progression of IPF. |
| Dales R, 2020 [75] | A longitudinal retrospective cohort study | Association between acute increases in air pollution exposures and risk of hospitalization in patients with IPF | Patients hospitalized with a primary diagnosis of IPF identified in the healthcare databases of the province of Santiago, Chile | Hospital admissions of patients with IPF were significantly higher on, or closely following days of higher air pollution. The most robust associations were noted for NO2 and PM10. Acute increases in air pollution are a risk factor for hospitalization of patients with IPF. |
| Johansson KA, 2014 [77] | A longitudinal prospective cohort study | Association between air pollution exposure and AE-IPF | 436 patients with IPF, South Korea | The risk of AE-IPF was significantly associated with an increased mean level of O3 (57% increased risk) and NO2 (41% increased risk) within the preceding 6 weeks. Increased exposure to air pollutants contributes to the development of AE-IPF. |
| Sesé L, 2018 [80] | A longitudinal prospective cohort study | Impact of air pollution exposures on the natural history of IPF | 192 patients with IPF, France | The risk of AE-IPF events was significantly associated with a higher mean concentration of O3 within the preceding 6 weeks with 47% increased risk per 10 µg/m3. Mortality was significantly associated with cumulative concentration of PM2.5 and PM10. Air pollution has a negative impact on the short and long-term outcomes in IPF. |
| Yoon HY, 2020 [81] | A longitudinal retrospective cohort study | Association between air pollution exposure and mortality in IPF | 1114 patients with IPF, South Korea | A 10-ppb increase in NO2 concentration was associated with a 17% increase in mortality of patients with IPF. Air pollution exposure can increase mortality in IPF. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski, S.; Piotrowski, W.J. Air Pollution—An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2021, 10, 77. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm10010077
Majewski S, Piotrowski WJ. Air Pollution—An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine. 2021; 10(1):77. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm10010077
Chicago/Turabian StyleMajewski, Sebastian, and Wojciech J. Piotrowski. 2021. "Air Pollution—An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis" Journal of Clinical Medicine 10, no. 1: 77. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm10010077






