Sustainable Management of Olive Orchard Nutrition: A Review
Abstract
:1. Introduction
2. Nitrogen, Phosphorus, and Potassium in Olive Orchards
3. Rain-Fed and Irrigated Olive Orchards
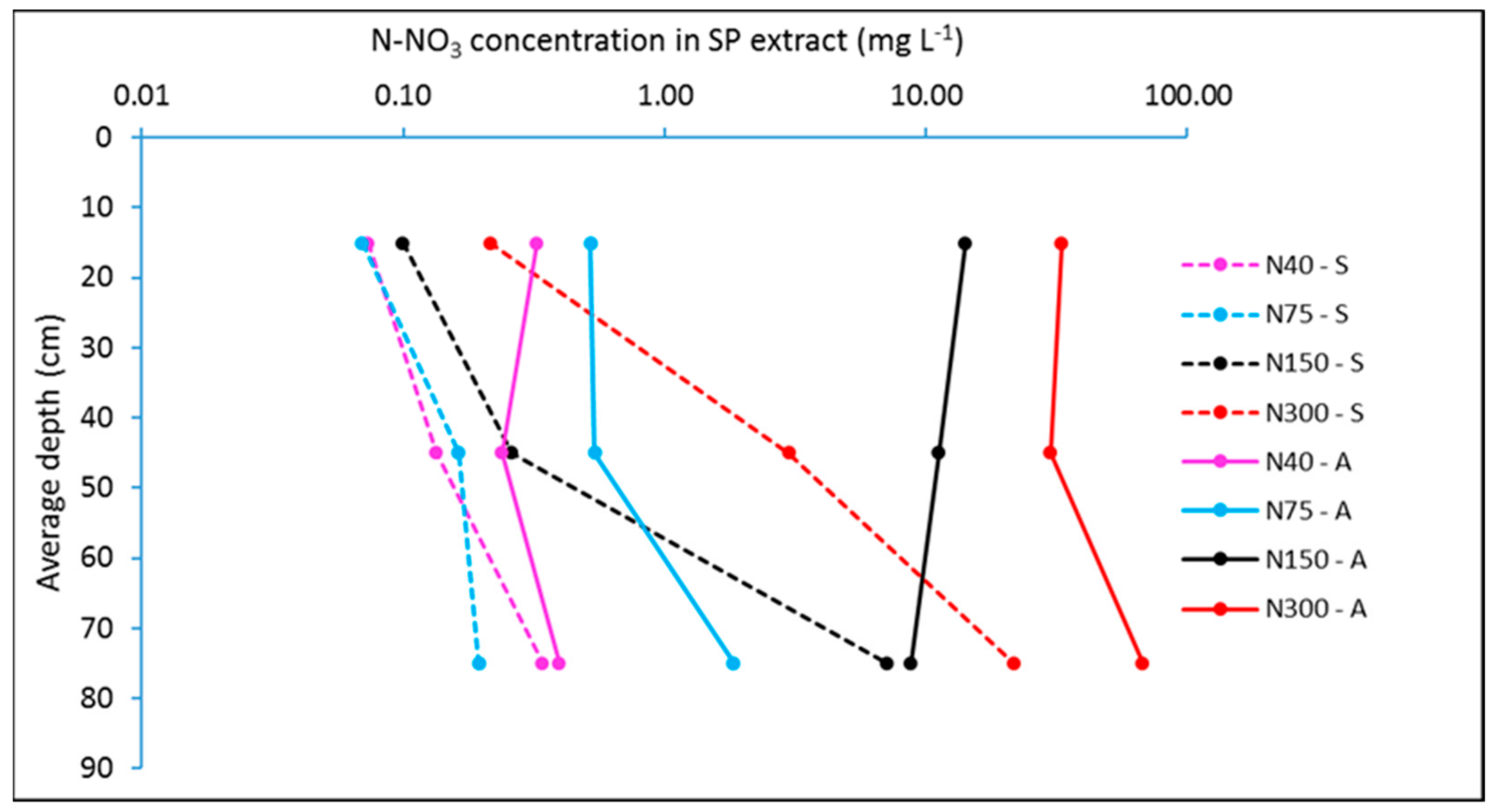
4. Nitrogen
5. Phosphorus
6. Potassium
7. Organic Material
8. Cover Crops
9. Recycling and Fertilization
10. Recycling of Olive Pomace and OMW
11. Irrigation with Recycled Wastewater as a Source of Nutrients
12. Effect of Soil pH on Selection of Fertilizers
13. Fertilization Management
14. Fertilization Management Criteria
15. Indirect Environmental Pollution
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy. 2017. Available online: http://www.fao.org/faostat/en/ (accessed on 10 December 2019).
- Rallo, L.; Caruzo, T.; Diez, C.M.; Campisi, G. Olive growing in a time of change: From empiricism to genomics. In The Olive Tree Genome; Rugini, L.B., Muleo, R., Sebastiani, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 55–64. [Google Scholar]
- Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Olive fertilization under intensive cultivation management. Acta Hortic. 2018, 1217, 207–224. [Google Scholar] [CrossRef]
- International Olive Council (IOC). World Olive Figures; International Olive Council (IOC): Madrid, Spain, 2016. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Fernandez-Escobar, R.; Moreno, R.; Sanchez-Zamora, M.A. Nitrogen dynamics in the olive bearing shoot. HortScience 2004, 19, 1406–1411. [Google Scholar] [CrossRef] [Green Version]
- Conor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: New York, NY, USA, 2004; Volume 31, pp. 155–229. [Google Scholar]
- Xiloyannis, C.; Celano, G.; Palese, A.; Dichio, B.; Nuzzo, V. Mineral nutrient uptake from the soil in irrigated olive trees, cultivar Coratina, over 6 years after planting. Acta Hortic. 2002, 586, 453–456. [Google Scholar] [CrossRef]
- Segal, E.; Dag, A.; Ben-Gal, A.; Zipori, I.; Erel, R.; Suryano, S.; Yermiyahu, U. Olive orchard irrigation with reclaimed wastewater: Agronomic and environmental considerations. Agric. Ecosyst. Environ. 2011, 140, 454–461. [Google Scholar] [CrossRef]
- Ben-Gal, A. Salinity and olive: From physiological responses to orchard management. Isr. J. Plant Sci. 2011, 59, 15–28. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Ferreira, I.Q.; Claro, A.M.; Arrobas, M. Fertilizer recommendations for olive based upon nutrients removed in crop and pruning. Sci. Hortic. 2012, 142, 205–211. [Google Scholar] [CrossRef]
- Zipori, I.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Response of oil-olive trees to iron application. Acta Hortic. 2011, 888, 295–300. [Google Scholar] [CrossRef]
- Zipori, I.; Yermiyahu, U.; Erel, R.; Presnov, E.; Faingold, I.; Ben-Gal, A.; Dag, A. The influence of irrigation level on olive tree nutritional status. Irrig. Sci. 2015, 33, 277–287. [Google Scholar] [CrossRef]
- Haberman, A.; Dag, A.; Shtern, N.; Zipori, I.; Erel, R.; Ben-Gal, A.; Yermiyahu, U. Significance of proper nitrogen fertilization for olive productivity in intensive cultivation. Sci. Hortic. 2019, 246, 710–717. [Google Scholar] [CrossRef]
- Dag, A.; Ben-David, E.; Kerem, Z.; Ben-Gal, A.; Erel, R.; Basheer, L.; Yermiyahu, U. Olive oil composition as a function of nitrogen, phosphorus and potassium plant nutrition. J. Sci. Food Agric. 2009, 89, 1871–1878. [Google Scholar] [CrossRef]
- Lam, S.K.; Suter, H.; Mosier, A.R.; Chen, D. Using nitrification inhibitors to mitigate agricultural N2O emission: A double-edged sword? Glob. Chang. Biol. 2017, 23, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.J.; Dag, A.; Raviv, M.; Zipori, I.; Medina, S.; Saadi, I.; Krasnovski, A.; Eizenberg, H.; Laor, Y. Annual spreading of olive mill wastewater over consecutive years: Effects on cultivated soils’ physical properties. Land Degrad. Dev. 2018, 29, 176–187. [Google Scholar] [CrossRef]
- Zipori, I.; Dag, A.; Laor, Y.; Levy, G.J.; Eizenberg, H.; Yermiyahu, U.; Medina, S.; Saadi, I.; Krasnovski, A.; Raviv, M. Potential nutritional value of olive-mill wastewater applied to irrigated olive (Olea europaea L.) orchard in a semi-arid environment over 5 years. Sci. Hortic. 2018, 241, 218–224. [Google Scholar] [CrossRef]
- Auerswald, K.; Kainz, M.; Angermüller, S.; Steindl, H. Influence of exchangeable potassium on soil erodibility. Soil Use Manag. 1996, 12, 117–121. [Google Scholar] [CrossRef]
- Chen, Y.; Banin, A.; Borochovitch, A. Effect of potassium on soil structure in relation to hydraulic conductivity. Geoderma 1983, 30, 135–147. [Google Scholar] [CrossRef]
- Fraser, A.I.; Harrod, T.R.; Haygarth, P.M. The effect of rainfall intensity on soil erosion and particulate phosphorus transfer from arable soils. Water Sci. Technol. 1999, 39, 41–45. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Dag, A.; Yermiyahu, U.; Tsipori, I.; Presnov, E.; Faingold, I.; Kerem, Z. Evaluation of irrigation in a converted, rain fed olive orchard: The transition year. Acta Hortic. 2006, 792, 99–106. [Google Scholar] [CrossRef]
- Lavee, S.; Schachtel, J. Interaction of cultivar rootstock and water availability on olive tree performance and fruit production. Acta Hortic. 1999, 474, 399–414. [Google Scholar] [CrossRef]
- Liu, Q.; Lan, Y.; Tan, F.; Tu, Y.; Sun, Y.; Yougu, G.; Yang, Z.; Ding, C.; Li, T. Drip irrigation elevated olive productivity in Southwest China. HortTechnology 2019, 29, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Therios, I. Mineral Nutrition of Olive Trees. In Proceedings of the 2nd International Seminar Recent Advances Olive Industry, Mazara del Vallo, Italy, 5–10 November 2006; pp. 403–410. [Google Scholar]
- López-Villalta, L.C.; Muñoz-Cobo, M.P. Production techniques. In World Olive Encyclopedia; International Olive Council: Sabadell, Spain, 1996; pp. 145–190. [Google Scholar]
- Ferreira, J.; Pastor, M.; Magallanes, M. Trials with mineral nutrition of olive tree’s leaves. In Seminaire sur L’olivier et Autres Plantes Oleagineuses Cultivees en Tunisie; Centre National de Documentation Agricole: Mahdia, Tunisia, 1978. [Google Scholar]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Toscano, P.; Godino, G.; Belfiore, T.; Bricolli-Bati, C. Foliar fertilization: A valid alternative for olive cultivar. Acta Hortic. 2002, 594, 191–195. [Google Scholar] [CrossRef]
- Restrepo-Diaz, H.; Benlloch, M.; Navarro, C.; Fernández-Escobar, R. Potassium fertilization of rainfed olive orchards. Sci. Hortic. 2008, 116, 399–403. [Google Scholar] [CrossRef]
- Restrepo-Diaz, H.; Benlloch, M.; Fernández-Escobar, R. Plant water stress and K+ starvation reduce absorption of foliar applied K+ by olive leaves. Sci. Hortic. 2008, 116, 409–413. [Google Scholar] [CrossRef]
- Bar-Yosef, B. Advances in fertigation. In Advances in Agronomy; Academic Press: New York, NY, USA, 1999; Volume 65, pp. 1–77. [Google Scholar]
- Neilsen, G.H.; Neilsen, D.; Peryea, F. Response of soil and irrigated fruit trees to fertigation or broadcast application of nitrogen, phosphorus, and potassium. HortTechnology 1999, 9, 393–401. [Google Scholar] [CrossRef]
- Kirkby, E. Introduction, Definition and Classification of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–5. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Use and abuse of nitrogen in olive fertilization. Acta Hortic. 2011, 888, 249–258. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Marin, L.; Sánchez-Zamora, M.A.; García-Novelo, J.M.; Molina-Soria, C.; Parra, M.A. Long-term effects of N fertilization on cropping and growth of olive trees and on N accumulation in soil profile. Eur. J. Agron. 2009, 31, 223–232. [Google Scholar] [CrossRef]
- Centeno, A.; García, J.M.; Gómez-del-Campo, M. Effects of nitrogen fertilization and nitrification inhibitor product on vegetative growth, production and oil quality in ’Arbequina’ hedgerow and ’Picual’ vase-trained orchards. Grasas Y Aceites 2017, 68, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.; García-Ortiz, A.; Frias, L.; Fernández, A. Los nutrientes N, P, K en la fertilización del olivar. Olea 1986, 17, 141–152. [Google Scholar]
- Hartmann, H.T. Some responses of the olive to nitrogen fertilizers. Proc. Am. Soc. Hortic. Sci. 1958, 72, 257–266. [Google Scholar]
- Morales-Sillero, A.; Fernández, J.E.; Ordovás, J.; Suárez, M.P.; Pérez, J.A.; Liñán, J.; López, E.P.; Girón, I.; Troncoso, A. Plant–soil interactions in a fertigated “Manzanilla de Sevilla” olive orchard. Plant Soil 2009, 319, 147–162. [Google Scholar] [CrossRef]
- Erel, R.; Yermiyahu, U.; Van Opstal, J.; Ben-Gal, A.; Schwartz, A.; Dag, A. The importance of olive (Olea europaea L.) tree nutritional status on its productivity. Sci. Hortic. 2013, 159, 8–18. [Google Scholar] [CrossRef]
- Erel, R.; Dag, A.; Ben-Gal, A.; Schwartz, A.; Yermiyahu, U. Flowering and fruit set of olive trees in response to nitrogen, phosphorus, and potassium. J. Am. Soc. Hortic. Sci. 2008, 133, 639–647. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Ortiz-Urquiza, A.; Prado, M.; Rapoport, H.F. Nitrogen status influence on olive tree flower quality and ovule longevity. Environ. Exp. Bot. 2008, 64, 113–119. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Finnemann, J.; Schjoerring, J.K. Translocation of NH4+ in oilseed rape plants in relation to glutamine synthetase isogene expression and activity. Physiol. Plant. 1999, 105, 469–477. [Google Scholar] [CrossRef]
- Erel, R.; Kerem, Z.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Zipori, I.; Yermiyahu, U. Olive (Olea europaea L.) tree nitrogen status is a key factor for olive oil quality. J. Agric. Food Chem. 2013, 61, 11261–11272. [Google Scholar] [CrossRef] [PubMed]
- Bustan, A.; Kerem, Z.; Yermiyahu, U.; Ben-Gal, A.; Lichter, A.; Droby, S.; Dag, A. Preharvest circumstances leading to elevated oil acidity in ’Barnea’ olives. Sci. Hortic. 2014, 176, 11–21. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use Manag. 2002, 18, 395–403. [Google Scholar] [CrossRef]
- Vilarrasa-Nogué, M.; Teira-Esmatges, M.R.; Villar, J.M.; Rufat, J. Effect of N dose on soil GHG emissions from a drip-fertigated olive (Olea europaea L.) orchard. Sci. Total Environ. 2019, 677, 350–361. [Google Scholar] [CrossRef]
- Freeman, M.; Uriu, K.; Hartmann, H.T. Diagnosing and correcting nutrient problems. In Olive Production Manual; Ferguson, L., Silbert, G.S., Martin, C.G., Eds.; University of California, Division of Agriculture and Resources: Oakland, CA, USA, 1994; pp. 77–86. [Google Scholar]
- Erel, R.; Yermiyahu, U.; Yasuor, H.; Chamus, D.C.; Schwartz, A.; Ben-Gal, A.; Dag, A. Phosphorous nutritional level, carbohydrate reserves and flower quality in olives. PLoS ONE 2016, 11, e0167591. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Moreno, M.J.; Fernández-Escobar, R. Response of young olive plants (Olea europaea) to phosphorus application. HortScience 2016, 51, 1167–1170. [Google Scholar] [CrossRef]
- Jiménez-Moreno, M.J.; Fernández-Escobar, R. Influence of nutritional status of phosphorus on flowering in the olive (Olea europaea L.). Sci. Hortic. 2017, 223, 1–4. [Google Scholar] [CrossRef]
- Dag, A.; Yermiyahu, U.; Ben-Gal, A.; Zipori, I.; Kapulnik, Y. Nursery and post-transplant field response of olive trees to arbuscular mycorrhizal fungi in an arid region. Crop Pasture Sci. 2009, 60, 427–433. [Google Scholar] [CrossRef]
- Estaún, V.; Camprubí, A.; Calvet, C.; Pinochet, J. Nursery and field response of olive trees inoculated with two arbuscular mycorrhizal fungi, Glomus intraradices and Glomus mosseae. J. Am. Soc. Hortic. Sci. 2003, 128, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Escobar, R. Mundi-prensa, junta de Andalucía. In Olive Growing, 1st ed.; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Australian Olive Association Ltd.: Pendle Hill, Australia, 2010. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Gómez, J.A.; Giráldez, J.V.; Pastor, M.; Fereres, E. Effects of tillage method on soil physical properties, infiltration and yield in an olive orchard. Soil Tillage Res. 1999, 52, 167–175. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Dudley, L.M. Phosphorus availability under continuous point source irrigation. Soil Sci. Soc. Am. J. 2003, 67, 1449–1456. [Google Scholar] [CrossRef]
- Klein, I.; Lavee, S. Effect of Nitrogen and Potassium Fertilizers on Olive Production. In Proceedings of the 13th Colloquium of the International Potash Institute, Coll York, UK, 1977; pp. 295–303. [Google Scholar]
- Bustan, A.; Avni, A.; Yermiyahu, U.; Ben-Gal, A.; Riov, J.; Erel, R.; Zipori, I.; Dag, A. Interactions between fruit load and macroelement concentrations in fertigated olive (Olea europaea L.) trees under arid saline conditions. Sci. Hortic. 2013, 152, 44–55. [Google Scholar] [CrossRef]
- Hussein, A.H.A. Response of Manzanillo olive (Olea europaea L.) cultivar to irrigation regime and potassium fertigation under tabouk conditions, Saudi Arabia. J. Agron. 2008, 7, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Beutel, J.; Uriu, K.; Lilleland, O. Leaf analysis for California deciduous fruits. In Soil and Plant Tissue Testing in California; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1983; pp. 15–17. [Google Scholar]
- Arquero, O.; Barranco, D.; Benlloch, M. Potassium starvation increases stomatal conductance in olive trees. HortScience 2006, 41, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [CrossRef] [Green Version]
- Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A.; Shapira, O.; Schwartz, A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J. Plant Physiol. 2015, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mimoun, M.B.; Loumi, O.; Ghrab, M.; Latiri, K.; Hellali, R. Foliar Potassium Application on Olive Tree. In Proceedings of the IPI Regional Workshop on Potassium and Fertigation Development in West Asia and North Africa, Rabat, Morocco, 24–28 November 2004. [Google Scholar]
- Kolenbrander, G.J. Fertilizers and Pollution. In Proceedings of the Transactions of the 12th International Congress of Soil Science Managing Soil Resources to Meet the Challenges to Mankind, The Indian Society of Soil Science, New Delhi, India, 8–16 February 1982; pp. 248–266. [Google Scholar]
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Larney, F.J.; Hao, X. A review of composting as a management alternative for beef cattle feedlot manure in southern Alberta, Canada. Bioresour. Technol. 2007, 98, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Bernal, M.P.; Roig, A. Composting olive mill waste and sheep manure for orchard use. Compost Sci. Util. 2004, 12, 130–136. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Sánchez-Monedero, M.A.; Roig, A.; López-Cano, I.; Moreno, B.; Benitez, E.; Cayuela, M.L. Compost vs biochar amendment: A two-year field study evaluating soil C build-up and N dynamics in an organically managed olive crop. Plant Soil 2016, 408, 1–14. [Google Scholar] [CrossRef]
- Tomati, U.; Galli, E.; Pasetti, L.; Volterra, E. Bioremediation of olive-mill wastewaters by composting. Waste Manag. Res. 1995, 13, 509–518. [Google Scholar] [CrossRef]
- Madejon, E.; Tomati, U.; Galli, E. Composting of wastes produced by low water consuming olive mill technology. Agrochimica 1998, 42, 135–146. [Google Scholar]
- Beltran, E.D.; Loresco, M.M.; Dizon, R.C.; Zuñiga, A.J.M. Waste management system at DTRI university farm: Utilization of manure, urine and farm residues as organic fertilizer at the dairy farm, its effects in the environment and sustainable livestock production system. In DTRI 2004 Annual Report (Philippines); University of the Philippines at Los Baños: Los Banos, Philippines, 2004. [Google Scholar]
- Whalen, J.K.; Hu, Q.; Liu, A. Compost applications increase water-stable aggregates in conventional and no-tillage systems. Soil Sci. Soc. Am. J. 2003, 67, 1842–1847. [Google Scholar] [CrossRef] [Green Version]
- Palese, A.M.; Vignozzi, N.; Celano, G.; Agnelli, A.E.; Pagliai, M.; Xiloyannis, C. Influence of soil management on soil physical characteristics and water storage in a mature rainfed olive orchard. Soil Tillage Res. 2014, 144, 96–109. [Google Scholar] [CrossRef]
- Dahan, O.; Babad, A.; Lazarovitch, N.; Russak, E.E.; Kurtzman, D. Nitrate leaching from intensive organic farms to groundwater. Hydrol. Earth Syst. Sci. 2014, 18, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Pinamonti, F.; Sicher, L. Compost utilization in fruit production systems. In Compost Utilization in Horticultural Cropping Systems; Lewis Publishers: New York, NY, USA, 2001; pp. 177–200. [Google Scholar]
- Watson, G.W.; Kelsey, P.; Woodtli, K. Replacing soil in the root zone of mature trees for better growth. J. Arboric. 1996, 22, 167–173. [Google Scholar]
- Kramer, S. Coping with soil limitation in the field: Variability, stones, salinity, at the Arava region, Israel. Acta Hortic. 2015, 1076, 45–52. [Google Scholar] [CrossRef]
- Dag, A.; Ben-Gal, A.; Yermiyahu, U.; Broner, M.; Hanoch, E.; Kerem, Z. The application of sludge compost in olive orchards. Report to Chief Scientist, Ministry of Agriculture and Rural Development, Israel in Hebrew. Available online: https://agriscience.co.il/docs/proposals/2436/203-0699-09.pdf (accessed on 10 December 2019).
- Li, X.; Niu, J.; Xie, B. The effect of leaf litter cover on surface runoff and soil erosion in Northern China. PLoS ONE 2014, 9, e107789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, O.M.; Fernández-Ondoño, E.; Castro, J. Sustainable agricultural practices for Mediterranean olive grove. Effect of soil management on soil properties. Span. J. Soil Sci. 2012, 2, 70–77. [Google Scholar] [CrossRef]
- Hernández, A.J.; Prieto, N.; Pastor Piñeiro, J. Management of an Olive Crop in a Semiarid Environment Using Sown or Resident Leguminous Covers. In Proceedings of the I World Congress on Conservation Agriculture, Madrid, Spain, 1–5 October 2001; Volume 25, pp. 419–423. [Google Scholar]
- Cerdan, O.; Govers, G.; Le Bissonnais, Y.; Van Oost, K.; Poesen, J.; Saby, N.; Gobin, A.; Vacca, A.; Quinton, J.; Auerswald, K.; et al. Rates and spatial variations of soil erosion in Europe: A study based on erosion plot data. Geomorphology 2010, 122, 167–177. [Google Scholar] [CrossRef]
- Renard, K.G.; Foster, G.R.; Weesies, G.A.; Porter, J.P. RUSLE: Revised universal soil loss equation. J. Soil Water Conserv. 1991, 46, 30–33. [Google Scholar]
- Ferreira, I.Q.; Arrobas, M.; Claro, A.M.; Rodrigues, M.A. Soil management in rainfed olive orchards may result in conflicting effects on olive production and soil fertility. Span. J. Agric. Res. 2013, 11, 472–480. [Google Scholar] [CrossRef]
- Gómez, J.A.; Guzmán, M.G.; Giráldez, J.V.; Fereres, E. The influence of cover crops and tillage on water and sediment yield, and on nutrient, and organic matter losses in an olive orchard on a sandy loam soil. Soil Tillage Res. 2009, 106, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.A. Sustainability using cover crops in Mediterranean tree crops, olives and vines—Challenges and current knowledge. Hung. Geogr. Bull. 2017, 66, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Pardini, A.; Faiello, C.; Snowball, R.; Mancuso, S.; Longhi, F. Cover crop species and their management in vineyards and olive groves. Adv. Hortic. Sci. 2002, 16, 225–234. [Google Scholar]
- Gómez-Muñoz, B.; Hatch, D.J.; Bol, R.; García-Ruiz, R. Nutrient dynamics during decomposition of the residues from a sown legume or ruderal plant cover in an olive oil orchard. Agric. Ecosyst. Environ. 2014, 184, 115–123. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Dimande, P.; Pereira, E.L.; Ferreira, I.Q.; Freitas, S.; Correia, C.M.; Muntinho-Perreira, J.; Arrobas, M. Early-maturing annual legumes: An option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosyst. 2015, 103, 153–166. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Correia, C.M.; Claro, A.M.; Ferreira, I.Q.; Barbosa, J.C.; Moutinho-Pereira, J.M.; Bachelar, E.A.; Fernandes-Silva, A.A.; Arrobas, M. Soil nitrogen availability in olive orchards after mulching legume cover crop residues. Sci. Hortic. 2013, 158, 45–51. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Walters, D.T.; Doran, J.W.; Francis, D.D.; Mosier, A.R. Crop residue type and placement effects on denitrification and mineralization. Soil Sci. Soc. Am. J. 1991, 55, 1020–1025. [Google Scholar] [CrossRef] [Green Version]
- Trinsoutrot, I.; Recous, S.; Bentz, B.; Lineres, M.; Cheneby, D.; Nicolardot, B. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- Turrini, A.; Caruso, G.; Avio, L.; Gennai, C.; Palla, M.; Agnolucci, M.; Tomei, P.E.; Giovannetti, M.; Gucci, R. Protective green cover enhances soil respiration and native mycorrhizal potential compared with soil tillage in a high-density olive orchard in a long term study. Appl. Soil Ecol. 2017, 116, 70–78. [Google Scholar] [CrossRef]
- Lavee, S. Biology and physiology of the olive. In World Olive Encyclopedia; International Olive Council: Madrid, Spain, 1996; p. 71. [Google Scholar]
- Weinbaum, S.A. Foliar nutrition of fruit trees. In Plant Growth and Leaf Applied Chemicals; CRC Press: Callaton, FL, USA, 1988; pp. 81–100. [Google Scholar]
- Repullo, M.A.; Carbonell, R.; Hidalgo, J.; Rodríguez-Lizana, A.; Ordóñez, R. Using olive pruning residues to cover soil and improve fertility. Soil Tillage Res. 2012, 124, 36–46. [Google Scholar] [CrossRef]
- Michailides, M.; Christou, G.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Composting of olive leaves and pomace from a three-phase olive mill plant. Int. Biodeterior. Biodegrad. 2011, 65, 560–564. [Google Scholar] [CrossRef]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef]
- Peikert, B.; Schaumann, G.E.; Keren, Y.; Bukhanovsky, N.; Borisover, M.; Garfha, M.; Shoqeir, J.H.; Dag, A. Characterization of topsoils subjected to poorly controlled olive oil mill wastewater pollution in West Bank and Israel. Agric. Ecosyst. Environ. 2015, 199, 176–189. [Google Scholar] [CrossRef]
- Buchmann, C.; Felten, A.; Peikert, B.; Munoz, K.; Bandow, N.; Dag, A.; Schaumann, G.E. Development of phytotoxicity and composition of soil treated with olive mill wastewater (OMW): An incubation study. Plant Soil 2015, 386, 99–112. [Google Scholar] [CrossRef]
- Saadi, I.; Laor, Y.; Raviv, M.; Medina, S. Land spreading of olive mill wastewater: Effects on soil microbial activity and potential phytotoxicity. Chemosphere 2007, 66, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Laor, Y.; Saadi, I.; Raviv, M.; Medina, S.; Erez-Reifen, D.; Eizenberg, H. Land spreading of olive mill wastewater in Israel: Current knowledge, practical experience, and future research needs. Isr. J. Plant Sci. 2011, 59, 39–51. [Google Scholar] [CrossRef]
- Grant, S.B.; Saphores, J.D.; Feldman, D.L.; Hamilton, A.J.; Fletcher, T.D.; Cook, P.L.M.; Stewardson, M.; Sanders, B.F.; Levin, L.A.; Ambrose, R.F.; et al. Taking the “waste” out of “wastewater” for human water security and ecosystem sustainability. Science 2012, 337, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyen, Z.; Moore, G.A.; Wrigley, R.J. Soil salinity and sodicity effects of wastewater irrigation in South East Australia. Agric. Water Manag. 2011, 99, 33–41. [Google Scholar] [CrossRef]
- Ayoub, S.; Al-Shdiefat, S.; Rawashdeh, H.; Bashabsheh, I. Utilization of reclaimed wastewater for olive irrigation: Effect on soil properties, tree growth, yield and oil content. Agric. Water Manag. 2016, 176, 163–169. [Google Scholar] [CrossRef]
- Levine, A.D.; Asano, T. Recovering sustainable water from wastewater. Environ. Sci. Technol. 2004, 38, 201A–208A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, A.J.; Stagnitti, F.; Xiong, X.; Kreidl, S.L.; Benke, K.K.; Maher, P. Wastewater irrigation: The state of play. Vadose Zone J. 2007, 6, 823–840. [Google Scholar] [CrossRef] [Green Version]
- Raveh, E.; Ben-Gal, A. Irrigation with water containing salts: Evidence from a macro-data national case study in Israel. Agric. Water Manag. 2016, 170, 176–179. [Google Scholar] [CrossRef]
- Erel, R.; Eppel, A.; Yermiyahu, U.; Ben-Gal, A.; Levy, G.; Zipori, I.; Schaumann, G.E.; Mayer, O.; Dag, A. Long-term irrigation with reclaimed wastewater: Implications on nutrient management, soil chemistry and olive (Olea europaea L.) performance. Agric. Water Manag. 2019, 213, 324–335. [Google Scholar] [CrossRef]
- Frenk, S.; Dag, A.; Yermiyahu, U.; Zipori, I.; Hadar, Y.; Minz, D. Seasonal effect and anthropogenic impact on the composition of the active bacterial community in Mediterranean orchard soil. FEMS Microbiol. Ecol. 2015, 91, fiv096. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Hadar, Y.; Minz, D. Resilience of soil bacterial community to irrigation with water of different qualities under Mediterranean climate. Environ. Microbiol. 2014, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Zipori, I.; Yermiyahu, U.; Tugendhaft, Y.; Ben-Gal, A.; Dag, A. The response of olive (Olea europaea) trees to zinc nutrition. Acta Hortic. 2016, 1199, 351–356. [Google Scholar] [CrossRef]
- Kafkafi, U.; Neumann, R.G. Correction of iron chlorosis in peanut (Arachis hypogea Shulamit) by ammonium sulfate and nitrification inhibitor. J. Plant Nutr. 1985, 8, 303–309. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating water stress in irrigated olives: Correlation of soil water status, tree water status, and thermal imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Fernández, J.E.; Perez-Martin, A.; Torres-Ruiz, J.M.; Cuevas, M.V.; Rodriguez-Dominguez, C.M.; Elsayed-Farag, S.; Morales-Sillero, A.; Garcia, J.M.; Hernandez-Santana, V.; Diaz-Espejo, A. A regulated deficit irrigation strategy for hedgerow olive orchards with high plant density. Plant Soil 2013, 372, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escobar, R.; Parra, M.A.; Navarro, C.; Arquero, O. Foliar diagnosis as a guide to olive fertilization. Span. J. Agric. Res. 2009, 7, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escobar, R.; de la Rosa, R.; León, L.; Gómez, J.A.; Testi, F.; Orgaz, M.; Gil-Ribes, J.A.; Quesada-Moraga, E.; Trapero, A. Evolution and sustainability of the olive production systems. In Present and Future of the Mediterranean Olive Sector—Options Méditerranéennes: Série A. Séminaires Méditerranéens; Arcas, N., Arroyo López, F.N., Caballero, J., D’Andria, R., Fernández, M., Fernandez Escobar, R., Garrido, A., López-Miranda, J., Msallem, M., Parras, M., et al., Eds.; CIHEAM: Zaragoza, Spain, 2013; Volume 106, pp. 11–41. [Google Scholar]







| Pruned Material | |||||||
| Year | Weight (kg ha−1) | Concentration in DM (%) | Amounts Removed (kg ha−1) | ||||
| N | P | K | N | P | K | ||
| 2014 | 10288 | 0.51 | 0.053 | 0.47 | 53.4 | 5.7 | 49.8 |
| 2015 | 7974 | 0.62 | 0.060 | 0.51 | 46.3 | 4.6 | 39.2 |
| 2016 | 13564 | 0.63 | 0.079 | 0.71 | 78.3 | 10.3 | 92.6 |
| Avg. | 10609 | 0.59 | 0.064 | 0.56 | 59.3 | 6.9 | 60.5 |
| Fruit | |||||||
| Year | Weight (kg ha−1) | Concentration in DM (%) | Amounts Removed (kg ha−1) | ||||
| N | P | K | N | P | K | ||
| 2011 | 6578 | 0.62 | 0.062 | 1.35 | 41.0 | 4.1 | 88.8 |
| 2012 | 6230 | 0.71 | 0.080 | 1.40 | 44.0 | 5.0 | 87.4 |
| 2013 | 7013 | 0.65 | 0.073 | 1.22 | 45.6 | 5.1 | 85.8 |
| 2014 | 5554 | 0.82 | 0.087 | 1.42 | 45.7 | 4.9 | 78.6 |
| 2015 | 3506 | 0.74 | 0.087 | 1.43 | 25.8 | 3.1 | 50.0 |
| Avg. | 5776 | 0.71 | 0.080 | 1.36 | 40.4 | 4.4 | 78.1 |
| Year | Avg. Yield (kg tree−1) | N (%) Young | N (%) Old | P (%) Young | P (%) Old | K (%) Young | K (%) Old |
|---|---|---|---|---|---|---|---|
| 2014 | 37.6 | 1.70a | 1.13b | 0.125A | 0.086B | 1.12A | 0.69B |
| 2015 | 25.0 | 1.57a | 1.46a | 0.132A | 0.123A | 1.17A | 1.19A |
| 2016 | 31.4 | 1.42a | 1.07b | 0.105A | 0.093A | 0.95A | 0.77B |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 11. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10010011
Zipori I, Erel R, Yermiyahu U, Ben-Gal A, Dag A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture. 2020; 10(1):11. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10010011
Chicago/Turabian StyleZipori, Isaac, Ran Erel, Uri Yermiyahu, Alon Ben-Gal, and Arnon Dag. 2020. "Sustainable Management of Olive Orchard Nutrition: A Review" Agriculture 10, no. 1: 11. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10010011





