Detecting Heat Stress in Dairy Cattle Using Neck-Mounted Activity Collars
Abstract
:1. Introduction
Automatic Detection of Heat Stress
2. Material and Methods
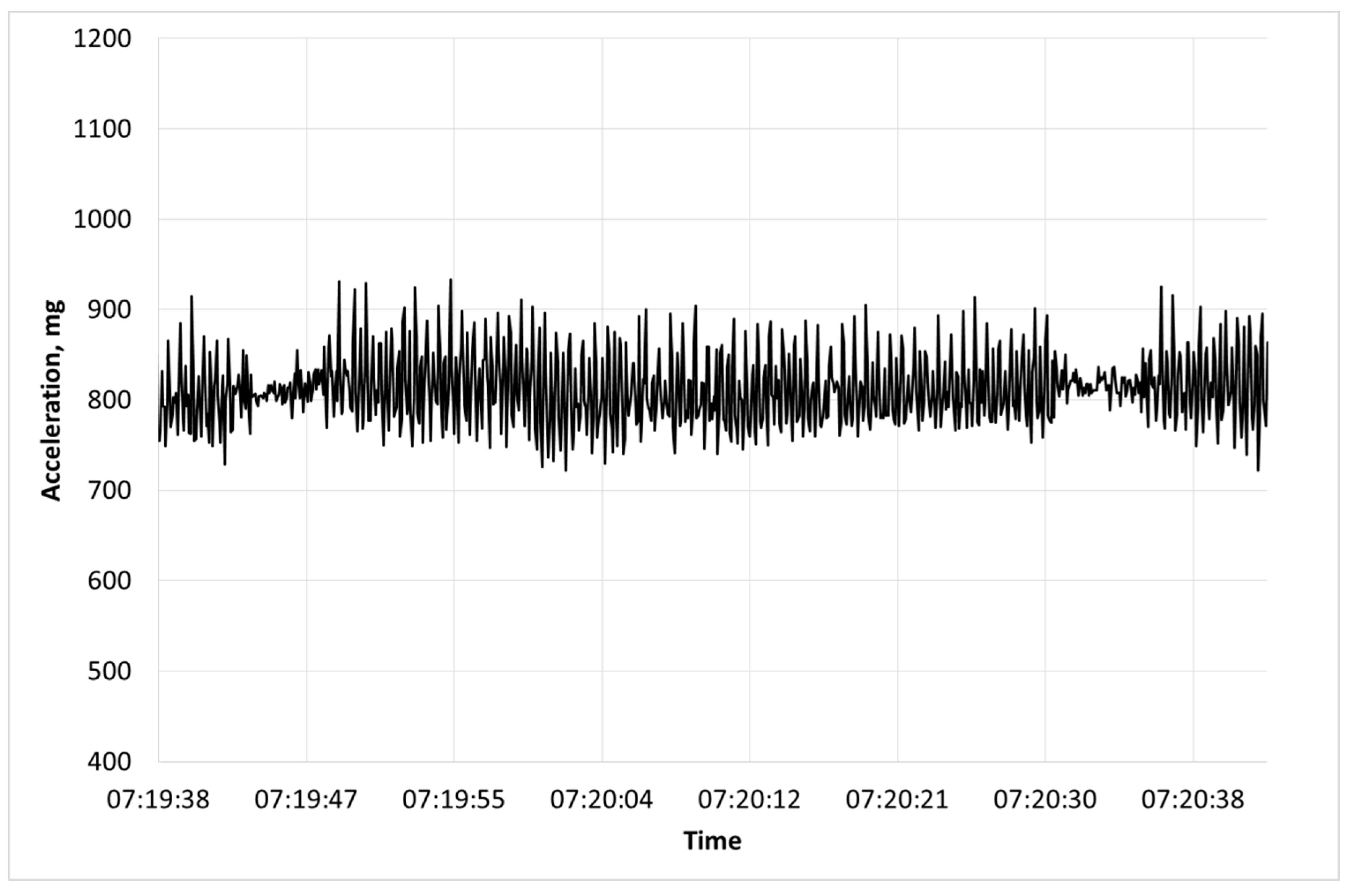
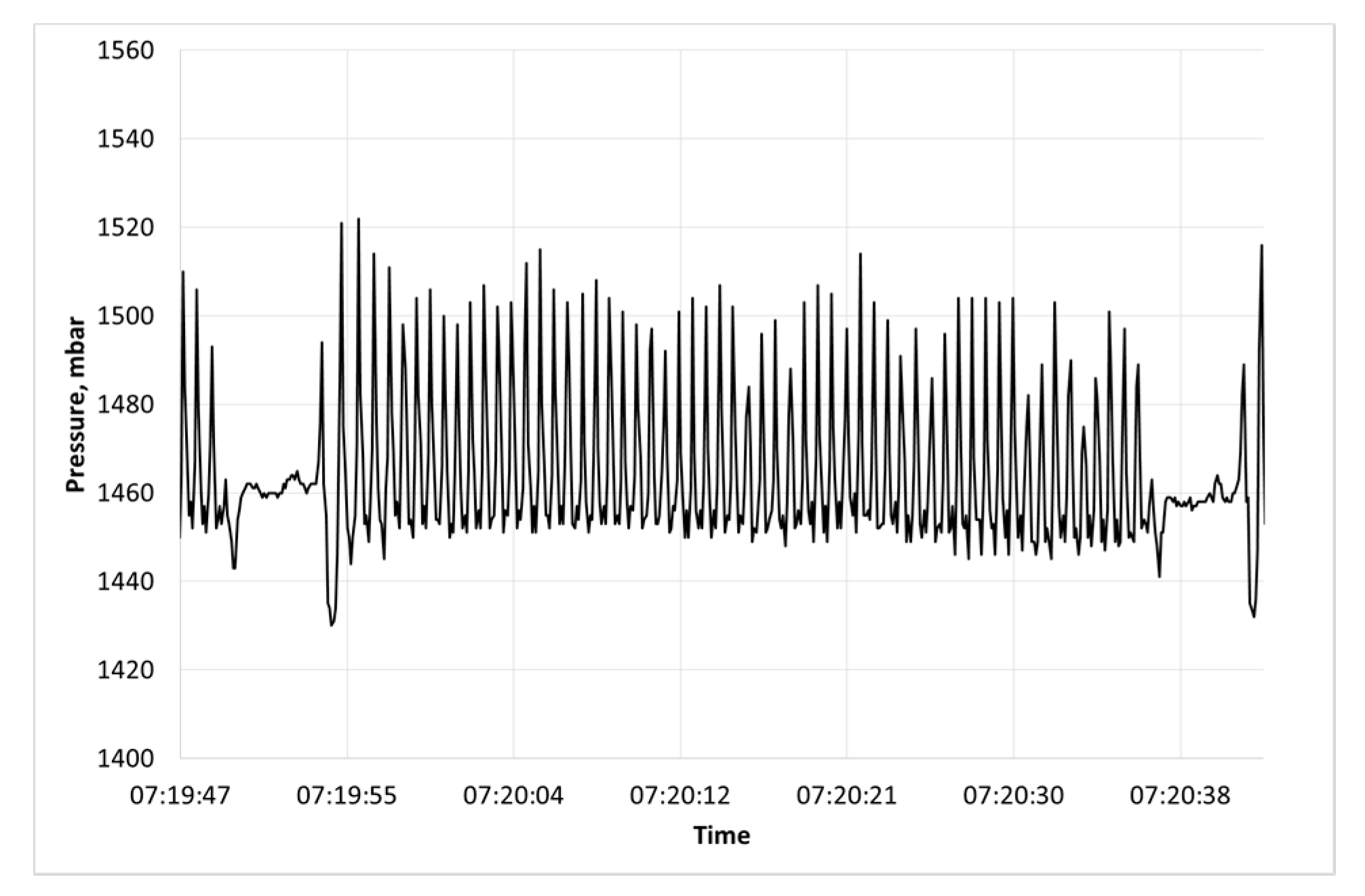
2.1. Accelerometer Based Collars
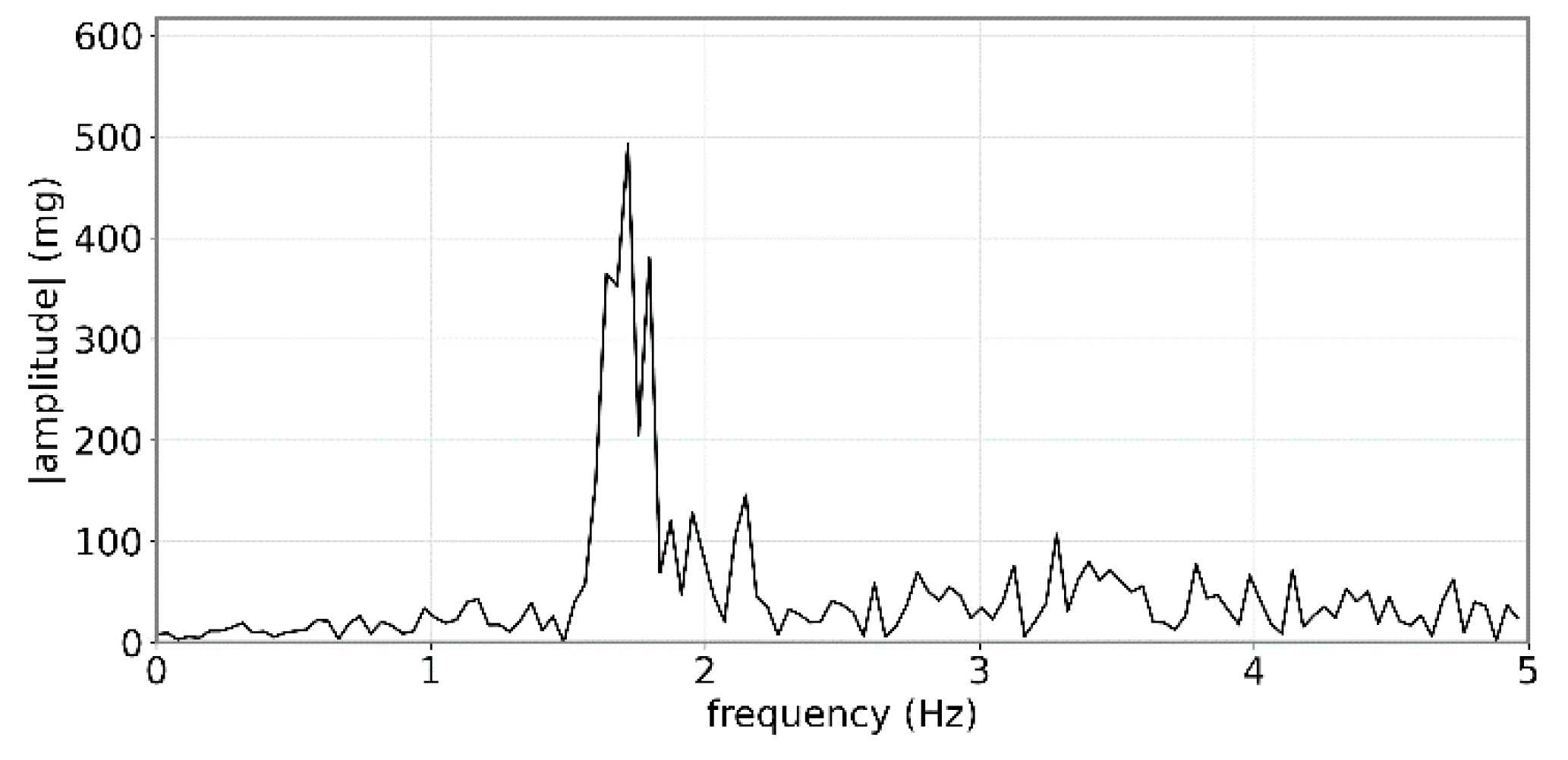
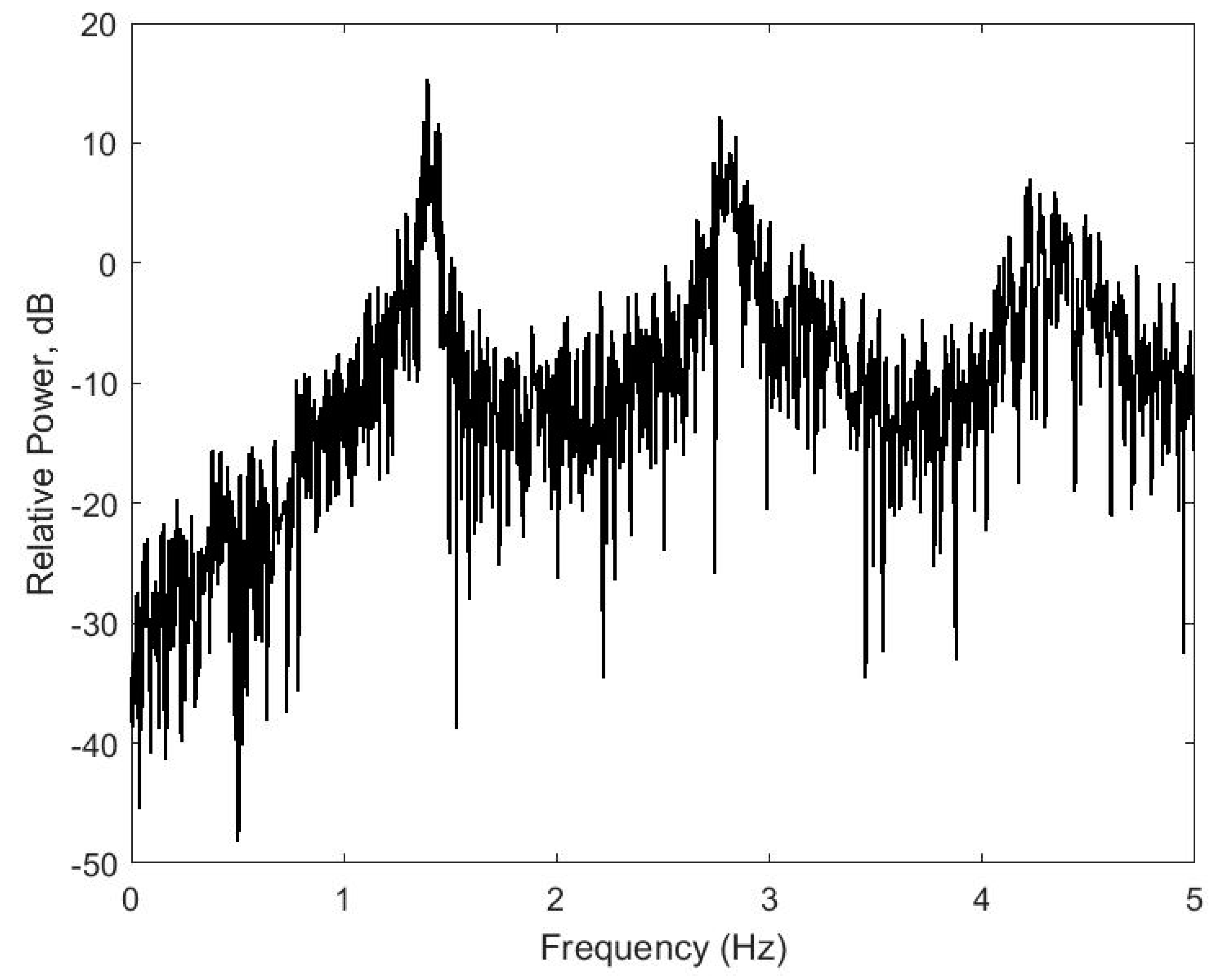
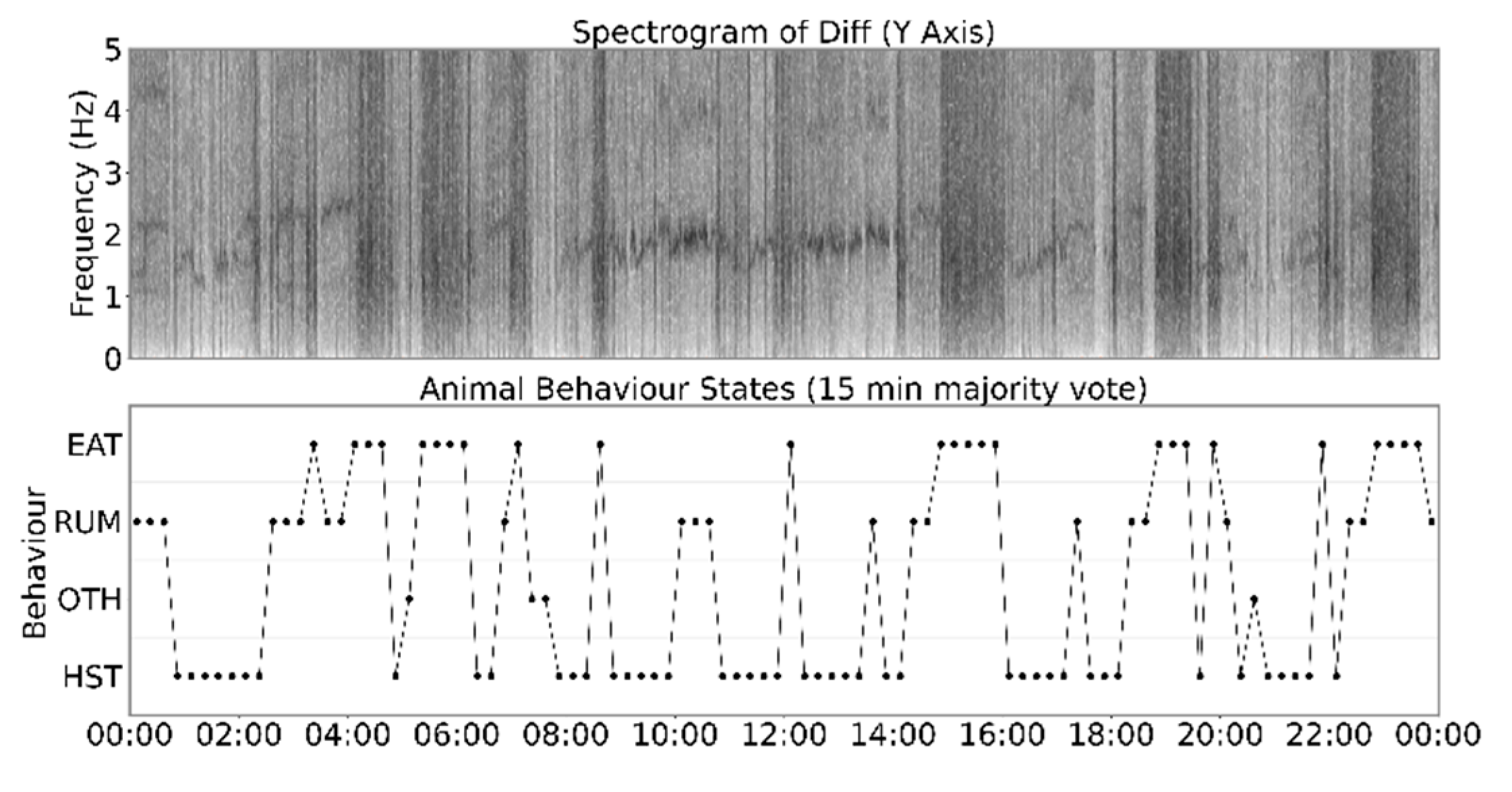
2.2. Heat Stress Signature
2.3. Comparison between Heat Stress Signature and Rumination
- Collect a 90s-long window of data;
- Calculate the energy measured by the accelerometer, , of the window;
- Calculate the Fourier Transform of the window;
- Extract the peak frequency value in the range 1–2 Hz;
- Normalise the peak amplitude to the spectrum mean in the range 1–2 Hz (F1–2);
- Extract the peak frequency value in the range 2–3 Hz;
- Normalise the peak amplitude to the spectrum mean in the range 2–3 Hz (F2–3);
- Classify the behaviour into other/rumination/eating, using (E, F2–3) and the method described in [30];
- Where rumination is identified, re-classify it as heat stress if F1–2 > F2–3.
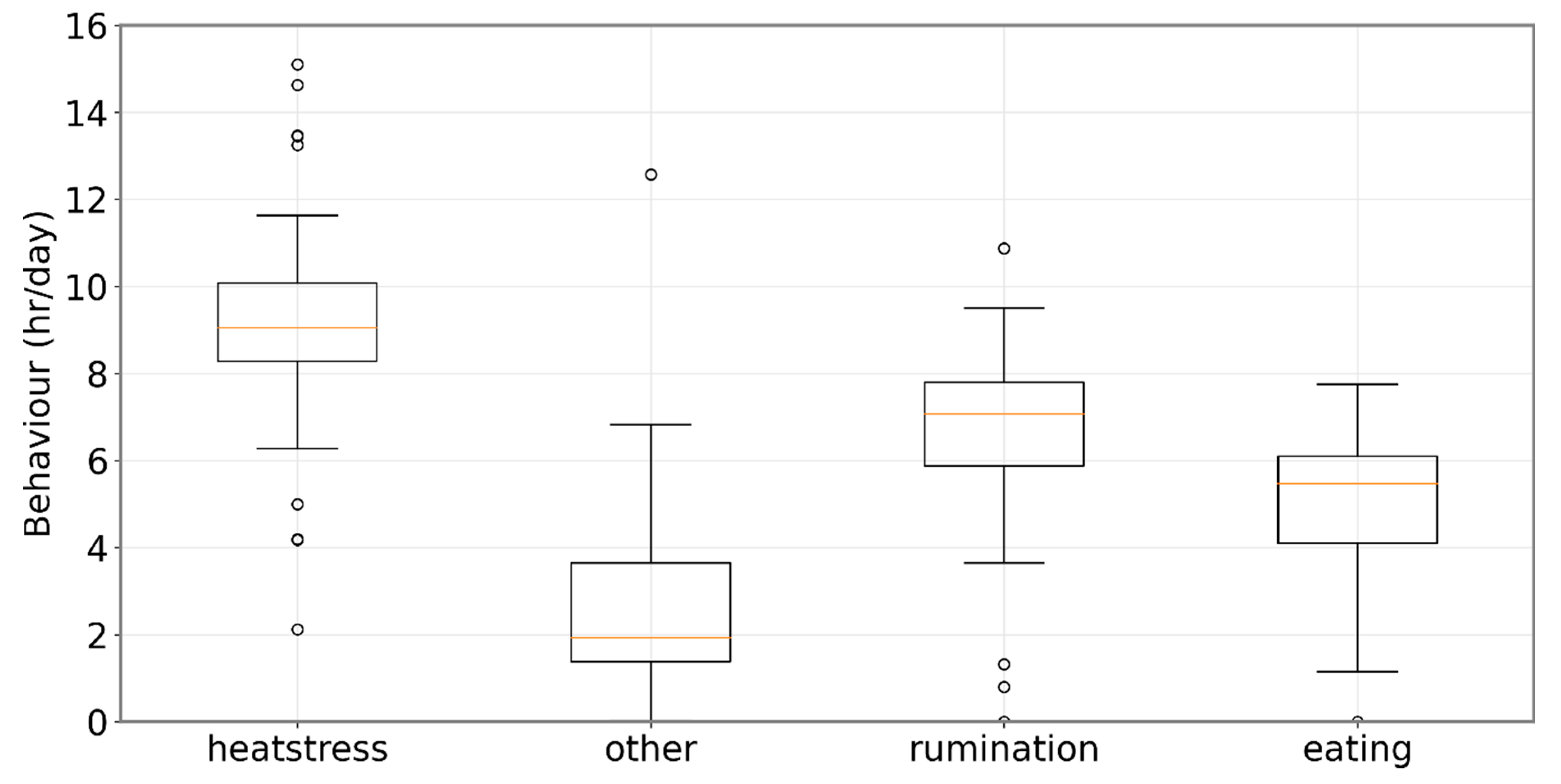
3. Results
Analysis of Measurement Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawkins, O. Dairy Industry in the UK: Statistics, Standard Note: SN/SG/2721. Available online: http://www.parliament.uk/briefing-papers/sn02721.pdf (accessed on 2 June 2020).
- NMR. Silent Herdsman. Available online: http://www.nmr.co.uk/silentherdsman (accessed on 2 June 2020).
- Fabdec. SenseHub™ the New Generation of Cow Monitoring. Available online: https://fabdec.com/wp-content/uploads/2019/07/GC_1193_Fabdec_SenseHub_210x210_web.pdf (accessed on 2 June 2020).
- Bar, D. Optimal Timing of Insemination Using Activity Collars. In Proceedings of the First North American Conference on Precision Dairy Management, Toronto, ON, Canada, 2–5 March 2010; Available online: http://precisiondairy.com/proceedings/s5bar.pdf (accessed on 1 June 2020).
- McGowan, J.E.; Burke, C.R.; Jago, J.G. Validation of a technology for objectively measuring behaviour in dairy cows and its application for oestrous detection. Proc. N. Z. Soc. Anim. Prod. 2007, 67, 136–142. [Google Scholar]
- Martiskainen, P.; Järvinen, M.; Skön, J.-K.; Tiirikainen, J.; Kolehmainen, M.; Mononen, J. Cow behaviour pattern recognition using a three-dimensional accelerometer and support vector machines. Appl. Anim. Behav. Sci. 2009, 119, 32–38. [Google Scholar] [CrossRef]
- Smith, D.; Rahman, A.; Bishop-Hurley, G.J.; Hills, J.; Shahriar, S.; Henry, D.; Rawnsley, R. Behavior classification of cows fitted with motion collars: Decomposing multi-class classification into a set of binary problems. Comput. Electron. Agric. 2016, 131, 40–50. [Google Scholar] [CrossRef]
- Robert, B.; White, B.J.; Renter, D.G.; Larson, R.L. Evaluation of three-dimensional accelerometers to monitor and classify behavior patterns in cattle. Comput. Electron. Agric. 2009, 67, 80–84. [Google Scholar] [CrossRef]
- Thorup, V.; Nielsen, B.; Robert, P.E.; Giger-Reverdin, S.; Konka, J.; Michie, C.; Friggens, N. Cow feeding and rumination behaviour can be characterized from sensor data to detect lameness. Front. Vet. Sci. 2016, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calamari, L.; Soriani, N.; Panella, G.; Petrera, F.; Minuti, A.; Trevisi, E. Rumination time around calving: An early signal to detect cows at greater risk of disease. J. Dairy Sci. 2014, 97, 3635–3647. [Google Scholar] [CrossRef] [PubMed]
- Büchel, S.; Sundrum, A. Short communication: Decrease in rumination time as an indicator of the onset of calving. J. Dairy Sci. 2014, 97, 3120–3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reith, S.; Hoy, S. Relationship between daily rumination time and estrus of dairy cows. J. Dairy Sci. 2012, 95, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.W. Design f large scale dairy cattle units in relation to management and animal welfare. In Knowledge Transfer in Cattle Husbandry; Kuipers, A., Klopcic, M., Thomas, C., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; Volume 117. [Google Scholar] [CrossRef]
- Koltes, J.E.; Koltes, D.A.; Mote, B.E.; Tucker, J.; Hubbell, D.S. Automated collection of heat stress data in livestock: New technologies and opportunities. Transl. Anim. Sci. 2018, 2, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Smaxtec Homepage. Available online: https://smaxtec.com/en/ (accessed on 2 June 2020).
- Lees, A.M.; Lees, J.C.; Lisle, A.T.; Sullivan, L.; Guaghan, J.B. Effect of heat stress on rumen temperature of three breeds of cattle. Int. J. Biometeorol. 2017, 62, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bergen, R.D.; Kennedy, A.D. Relationship between vaginal and tympanic membrane temperature in beef heifers. Can. J. Anim. Sci. 2000, 80, 515–518. [Google Scholar] [CrossRef]
- Davis, J.D.; Vanzant, E.S.; Purswell, J.L.; Green, A.R.; Bicudo, J.R.; Gates, R.S.; Holloway, L.E.; Smith, W.T. Methods of Remote, Continuous Temperature Detection in Beef Cattle. In Proceedings of the ASAE Annual International Meeting, Las Vegas, NV, USA, 27–30 July 2003. [Google Scholar] [CrossRef]
- Lee, Y.; Bok, J.D.; Lee, H.J.; Lee, H.G.; Kim, D.; Lee, I.; Kanf, S.K.; Choi, Y.J. Body Temperature Monitoring Using Subcutaneously Implanted Thermo-loggers from Holstein Steers. Asian-Australas. J. Anim. Sci. 2016, 29, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, J.D.; Saxton, A.M.; Ríus, A.G. Relationships among temperature-humidity index with rectal, udder surface, and vaginal temperatures in lactating dairy cows experiencing heat stress. J. Dairy Sci. 2018, 101, 6424–6429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunc, P.; Knížková, I. The use of infrared thermography in livestock production and veterinary field. In Infrared Thermography Recent Advances and Future Trends; Meola, C., Ed.; Bentham Books: Sharjah, United Arab Emirates, 2012; pp. 85–101. [Google Scholar] [CrossRef] [Green Version]
- Rural Chemical Industries. How to Spot Heat Stressed Beef Cattle. Available online: https://www.heatstress.info/heatstressinfo/Heatstressincattlepoultryandswine/HeatStressedBeefcattle/tabid/2202/Default.aspx (accessed on 1 June 2020).
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaughan, J.B.; Holt, S.M.; Hahn, G.L.; Mader, T.L.; Eigenberg, R. Respiration Rate—Is It a Good Measure of Heat Stress in Cattle? Asian-Australas. J. Anim. Sci. 2000, 13 (Suppl. C), 329–332. [Google Scholar]
- Ohnstad, I. Managing Heat Stress in Dairy Cows. 2012. National Animal Disease Information Service. Available online: https://www.nadis.org.uk/disease-a-z/cattle/managing-heat-stress-in-dairy-cows/ (accessed on 2 June 2020).
- STMicroelectronics. LIS3DH. Available online: https://www.st.com/en/mems-and-sensors/lis3dh.html (accessed on 2 June 2020).
- Analog Devices. Accelerometers. Available online: https://www.analog.com/en/products/sensors-mems/accelerometers.html (accessed on 2 June 2020).
- Braun, U.; Trösch, L.; Nydegger, F.; Hässig, M. Evaluation of eating and rumination behaviour in cows using a noseband pressure sensor. BMC Vet. Res. 2013, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlatte, T.W. Temperature-humidity index. In Climatology. Encyclopedia of Earth Science; Springer: Boston, MA, USA, 1987; pp. 837–838. [Google Scholar] [CrossRef]
- Michie, C.; Andonovic, I.; Tachtatzis, C.; Davison, C.; Konka, J. Wireless MEMS sensors for precision farming. In Wireless MEMS Networks and Applications; Uttamchandani, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 215–238. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davison, C.; Michie, C.; Hamilton, A.; Tachtatzis, C.; Andonovic, I.; Gilroy, M. Detecting Heat Stress in Dairy Cattle Using Neck-Mounted Activity Collars. Agriculture 2020, 10, 210. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10060210
Davison C, Michie C, Hamilton A, Tachtatzis C, Andonovic I, Gilroy M. Detecting Heat Stress in Dairy Cattle Using Neck-Mounted Activity Collars. Agriculture. 2020; 10(6):210. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10060210
Chicago/Turabian StyleDavison, Christopher, Craig Michie, Andrew Hamilton, Christos Tachtatzis, Ivan Andonovic, and Michael Gilroy. 2020. "Detecting Heat Stress in Dairy Cattle Using Neck-Mounted Activity Collars" Agriculture 10, no. 6: 210. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10060210






