Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.)
Abstract
:1. Introduction
2. Materials and Methods
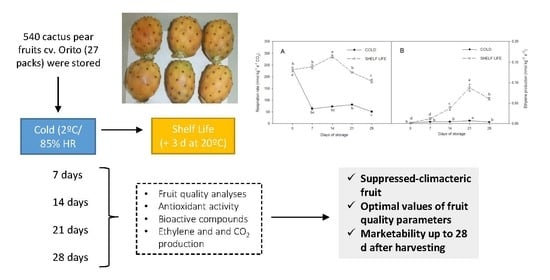
2.1. Plant Material and Experimental Design
2.2. Ethylene Production and Respiration Rate
2.3. Fruit Quality Parameters
2.4. Antioxidant Activity and Bioactive Compounds
2.5. Statistical Analyses
3. Results and Discussion
3.1. Ethylene and CO2 Production
3.2. Fruit Quality Parameters
3.3. Bioactive Compounds and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiesling, R. Origen, domesticación y distribución de Opuntia ficus-indica. J. Prof. Assoc. Cactus Dev. 1998, 3, 50–59. [Google Scholar]
- FAO (Food and Agricultural Organization of the United Nations). Ecologia del Cultivo, Manejo y Usos del Nopal; Inglese, P., Mondragon, C., Nefzaoui, A., Sáenz, C., Eds.; FAO: Rome, Italy, 2018; ISBN 9789251304945. [Google Scholar]
- Pimienta-Barrios, E. Prickly Pear (Opuntia spp.): A valuable fruit crop for the semi-arid lands of Mexico. J. Arid Environ. 1994, 28, 1–11. [Google Scholar] [CrossRef]
- FAO (Food and Agricultural Organization of the United Nations). Utilización Agroindustrial del Nopal. Agricultural Services Bulletin 162; Sáenz, C., Ed.; FAO: Rome, Italy, 2006. [Google Scholar]
- Reyes-Agüero, J.A.; Aguirre, J.R.; Carlín-Castelán, F.; González-Durán, A. Diversity of wild and cultivated opuntia variants in the meridional highlands plateau of Mexico. Acta Hortic. 2013, 995, 69–74. [Google Scholar] [CrossRef]
- Hahn-Schlam, F.; Valle-Guadarrama, S.; Jenkins, T. Robotic cactus pear cryocauterization increases storage life. Postharvest Biol. Technol. 2019, 147, 132–138. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Mitchell, F. Postharvest Handling Systems: Small Fruit (Table Grapes, Strawberries, Kiwifruit). In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; Publication 3311; University of California Division of Agriculture and Natural Resources: Oakland, CA, USA, 1992; pp. 357–374. [Google Scholar]
- Allegra, A.; Sortino, G.; Miciletta, G.; Riotto, M.; Fasciana, T.; Inglese, P. The influence of harvest period and fruit ripeness at harvest on minimally processed cactus pears (Opuntia ficus-indica L. Mill.) stored under passive atmosphere. Postharvest Biol. Technol. 2015, 104, 57–62. [Google Scholar] [CrossRef]
- Cruz-Bravo, R.K.; Guzmán-Maldonado, S.H.; Araiza-Herrera, H.A.; Zegbe, J.A. Storage alters physicochemical characteristics, bioactive compounds and antioxidant capacity of cactus pear fruit. Postharvest Biol. Technol. 2019, 150, 105–111. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Effects of UVB Light, Wounding Stress, and Storage Time on the Accumulation of Betalains, Phenolic Compounds, and Ascorbic Acid in Red Prickly Pear (Opuntia ficus-indica cv. Rojo Vigor). Food Bioprocess Technol. 2018, 11, 2265–2274. [Google Scholar] [CrossRef]
- Zegbe, J.A.; Serna-Pérez, A.; Mena-Covarrubias, J. Irrigation enhances postharvest performance of “Cristalina” cactus pear fruit. Acta Hortic. 2015, 1067, 417–422. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M. Bioactive Compounds, Antioxidant Activity and Quality of Plum and Sweet Cherry Cultivars as Affected by Ripening on-tree, Cold Storage and Postharvest Treatments. Ph.D. Thesis, Universidad Miguel Hernández de Elche, Elche, Spain, 2011. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Addinsoft, S.A.R.L. XLSTAT Software 2010; Addinsoft: Barcelona, Spain, 2010. [Google Scholar]
- Systat Software. SigmaPlot for Windows, Version 11.0; Systat Software: San Jose, CA, USA, 2008. [Google Scholar]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantwell, M. Post-Harvest Management of Fruits and Vegetable Stems; FAO: Roma, Italy, 1995; pp. 120–141. [Google Scholar]
- Lakshminarayana, S.; Estrella, I.B. Postharvest Respiratory Behaviour of Tuna (Prickly Pear) Fruit (Opuntia robusta Mill.). J. Hortic. Sci. 1978, 53, 327–330. [Google Scholar] [CrossRef]
- Zuzunaga, M.; Serrano, M.; Martínez-Roero, D.; Valero, D.; Riquelme, F. Comparative study of two plum (Prunus salicina Lindl.) cultivars during growth and ripening. Food Sci. Technol. Int. 2001, 7, 123–130. [Google Scholar] [CrossRef]
- Minas, I.S.; Font i Forcada, C.; Dangl, G.S.; Gradziel, T.M.; Dandekar, A.M.; Crisosto, C.H. Discovery of non-climacteric and suppressed climacteric bud sport mutations originating from a climacteric Japanese plum cultivar (Prunus salicina Lindl.). Front. Plant. Sci. 2015, 6, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirra, M.; Inglese, P.; La Mantia, T. Quality of cactus pear [Opuntia ficus-indica (L.) Mill.] fruit in relation to ripening time, CaCl2 pre-harvest sprays and storage conditions. Sci. Hortic. 1999, 81, 425–436. [Google Scholar] [CrossRef]
- Cantwell, M.; Rodríguez-Félix, A.; Robles-Contreras, F. Postharvest physiology of prickly pear cactus stems. Sci. Hortic. 1992, 50, 1–9. [Google Scholar] [CrossRef]
- Corrales-García, J.; Andrade-Rodríguez, J.; Bernabé-Cruz, E. Response of six cultivars of tuna fruits to cold storage. J. Prof. Assoc. Cactus Dev. 1997, 2, 160–168. [Google Scholar]
- D’Aquino, S.; Chessa, I.; Schirra, M. Heat Treatment at 38 °C and 75–80% Relative Humidity Ameliorates Storability of Cactus Pear Fruit (Opuntia ficus-indica cv “Gialla”). Food Bioprocess Technol. 2014, 7, 1066–1077. [Google Scholar] [CrossRef]
- Lamúa, M. Aplicación de Frío en Los Alimentos; Ediciones Mundiprensa: Madrid, Spain, 2000; Chapters 2, 3 and 7. [Google Scholar]
- López-Castañeda, J.; Corrales-García, J.; Terrazas-Salgado, T.; Colinas-León, T. Effect of vapor heat treatments on weight loss reduction and epicuticular changes in six varieties of cactus pear fruit (Opuntia spp.). J. Prof. Assoc. Cactus Dev. 2010, 12, 37–47. [Google Scholar]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC-Taylor and Francis: Boca Raton, FL, USA, 2010; pp. 35–230. ISBN 9781439802663. [Google Scholar]
- Hernández, F.; Andreu-Coll, L.; Cano-Lamadrid, M.; López-Lluch, D.; Carbonell-Barrachina, Á.A.; Legua, P. Valorization of Prickly Pear [Opuntia ficus-indica (L.) Mill]: Nutritional Composition, Functional Properties and Economic Aspects. In Cactaceae-Current Trends and Future Perspectives; IntechOpen: Rijeka, Croatia, 2020; pp. 1–5. [Google Scholar]
- Andreu-Coll, L.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Volatile composition of prickly pear fruit pulp from six Spanish cultivars. J. Food Sci. 2020, 85, 358–363. [Google Scholar] [CrossRef]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Graça-Miguel, M.; Gago, C.; Valente, R.; Guerreiro, A.; Antunes, D.; Manhita, A.; Barrocas-Dias, C. Qualitative evaluation of fruits from different Opuntia ficus-indica ecotypes/cultivars harvested in South Portugal. J. Food Biochem. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Guerrero-Beltrán, J.Á. The effects of modified atmospheres on prickly pear (Opuntia albicarpa) stored at different temperatures. Postharvest Biol. Technol. 2016, 111, 314–321. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crop. Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coria-Cayupán, Y.S.; Ochoa, M.J.; Nazareno, M.A. Health-promoting substances and antioxidant properties of Opuntia sp. fruits. Changes in bioactive-compound contents during ripening process. Food Chem. 2011, 126, 514–519. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Abd El-Hady, E.S.A.; Omran, H.T.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res. Int. 2014, 64, 864–872. [Google Scholar] [CrossRef]
- Ramírez-Ramos, M.; García-Mateos, M.; Corrales-García, J.; Ybarra-Moncada, C.; Castillo-González, A.M. Compuestos antioxidantes en variedades pigmentadas de tuna (Opuntia sp.). Rev. Fitotec. Mex. 2015, 38, 349–357. [Google Scholar] [CrossRef]
- Anorve Morga, J.; Aquino Bolanos, E.N.; Mercado Silva, E. Effect of Storage Temperature on Quality of Minimally Processed Cactus Pear. Acta Hortic. 2006, 217–222. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biol. Technol. 2009, 51, 354–363. [Google Scholar] [CrossRef]
- Reche, J.; García-Pastor, M.E.; Valero, D.; Hernández, F.; Almansa, M.S.; Legua, P.; Amorós, A. Effect of modified atmosphere packaging on the physiological and functional characteristics of Spanish jujube (Ziziphus jujuba Mill.) cv “Phoenix” during cold storage. Sci. Hortic. 2019, 258, 108743. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Plaza, L.; Crespo, I.; de Pascual-Teresa, S.; de Ancos, B.; Sánchez-Moreno, C.; Muñoz, M.; Cano, M.P. Impact of minimal processing on orange bioactive compounds during refrigerated storage. Food Chem. 2011, 124, 646–651. [Google Scholar] [CrossRef]




| Days of Storage | 0 | 7 | 14 | 21 | 28 | 7 + SL | 14 + SL | 21 + SL | 28 + SL |
|---|---|---|---|---|---|---|---|---|---|
| TSS (%) | 14.9 | 14.8 | 14.5 | 14.3 | 14.5 | 14.1 | 14.5 | 14.4 | 14.0 |
| TA (g malic acid kg−1) | 0.9 | 0.8 | 0.8 | 0.9 | 0.9 | 0.8 | 0.9 | 0.9 | 0.9 |
| Ripening index | 168 b | 185 a | 180 a | 160 b | 161 b | 176 a | 161 b | 160 b | 155 b |
| Weight loss (%) | 0 g | 0.28 f | 1.05 e | 1.97 c,d | 2.22 b,c | 1.13 e | 1.82 d | 2.43 b | 3.71 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu-Coll, L.; García-Pastor, M.E.; Valero, D.; Amorós, A.; Almansa, M.S.; Legua, P.; Hernández, F. Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.). Agriculture 2021, 11, 62. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11010062
Andreu-Coll L, García-Pastor ME, Valero D, Amorós A, Almansa MS, Legua P, Hernández F. Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.). Agriculture. 2021; 11(1):62. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11010062
Chicago/Turabian StyleAndreu-Coll, Lucía, María Emma García-Pastor, Daniel Valero, Asunción Amorós, María Soledad Almansa, Pilar Legua, and Francisca Hernández. 2021. "Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.)" Agriculture 11, no. 1: 62. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11010062