Modified Atmospheric Packaging of Fresh-Cut Amaranth (Amaranthus tricolor L.) for Extending Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment Method of Amaranth and Experimental Setup
2.2. Sensory Properties
2.3. Weight Loss Rate
2.4. Water Distribution and Migration
2.5. Soluble Solids
2.6. Chlorophyll Content
2.7. Aerobic Plate Count and Specific Spoilage Organism (SSO) Count
2.8. Ascorbic Acid (AsA) Content
2.9. Malondialdehyde (MDA) Content
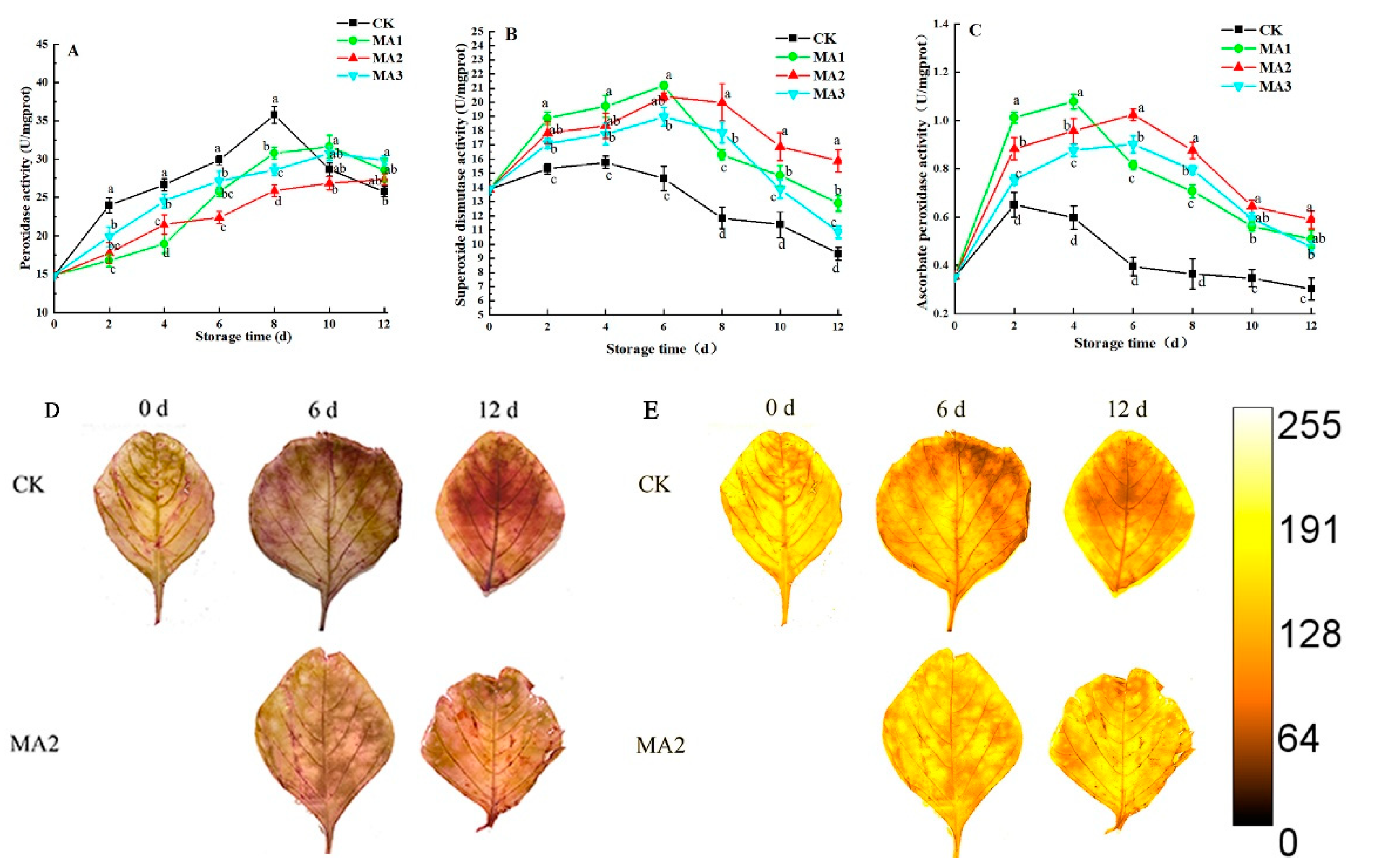
2.10. Peroxidase (POD) Activity
2.11. Superoxide Dismutase (SOD) Activity
2.12. Ascorbate Peroxidase (APX) Activity
2.13. NBT Staining
2.14. Data Analysis
3. Results and Discussion
3.1. Changes in Sensory Properties
3.2. Changes in Weight Loss and Water Migration
3.3. Changes in Soluble Solid Content
3.4. Changes in Chlorophyll Content
3.5. Aerobic Plate Count and SSO Count
3.6. Changes in AsA Content and MDA Content
3.7. Changes in Antioxidant Enzyme Activity
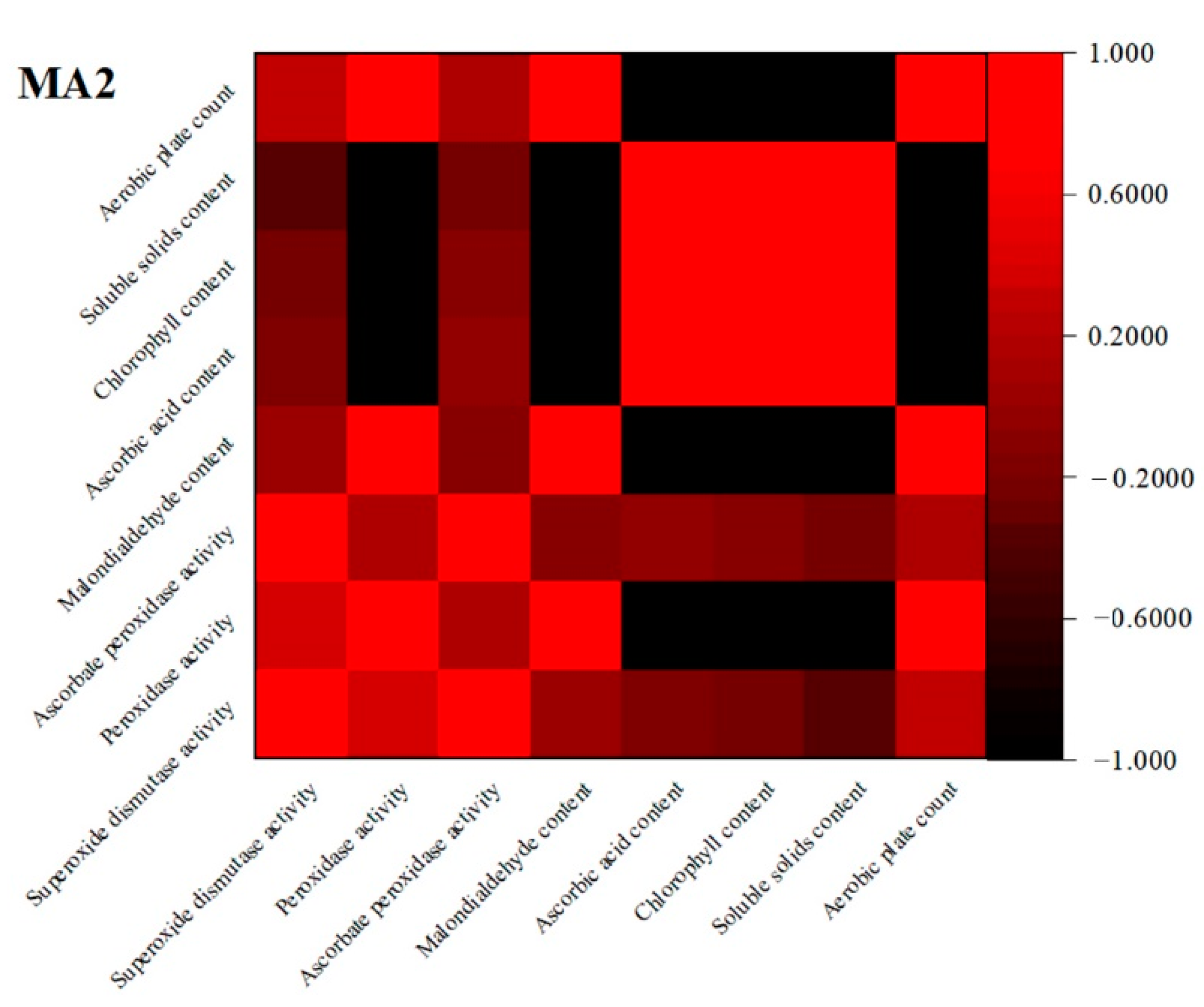
3.8. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gins, M.; Gins, V.; Motyleva, S.; Kulikov, I.; Medvedev, S.; Pivovarov, V.; Mertvishcheva, M. Metabolites with antioxidant and protective functions from leaves of vegetable amaranth (Amaranthus tricolor L.). Agric. Biol. 2017, 52, 1030–1040. [Google Scholar] [CrossRef]
- György, É.; Laslo, É.; Kuzman, I.H.; Dezső András, C. The effect of essential oils and their combinations on bacteria from the surface of fresh vegetables. Food Sci. Nutr. 2020, 8, 5601–5611. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Bhandari, B.; Jiang, F. A combination treatment of ultrasound and ε-polylysine to improve microorganisms and storage quality of fresh-cut lettuce. LWT 2019, 113, 108315. [Google Scholar] [CrossRef]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological and sensory quality of small berry fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Mudau, A.R.; Soundy, P.; Araya, H.T.; Mudau, F.N. Influence of modified atmosphere packaging on postharvest quality of baby spinach (Spinacia oleracea L.) leaves. HortScience 2018, 53, 224–230. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Ragaert, P.; Haque, M.A.; Eriksson, M.; van Labeke, M.C.; Devlieghere, F. High oxygen atmospheres can induce russet spotting development in minimally processed iceberg lettuce. Postharvest Biol. Technol. 2015, 100, 168–175. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anjum, M.A.; Nawaz, A.; Shah, H.M.S. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Artès, F.; Gómez, P.; Artés-Hernández, F. Physical, physiological and microbial deterioration of minimally fresh processed fruits and vegetables. Food Sci. Technol. Int. 2007, 13, 177–188. [Google Scholar] [CrossRef]
- Song, Y.S.; Stewart, D.; Reineke, K.; Wang, L.; Ma, C.; Lu, Y.; Shazer, A.; Deng, K.; Tortorello, M.L. Effects of package atmosphere and storage conditions on minimizing risk of escherichia coli O157: H7 in packaged fresh baby spinach. J. Food Prot. 2019, 82, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, Y.; Ma, Y.; Tong, J.; Zhang, J.; Zhang, Y.; Liang, H.; Zhao, X. Microbiological and physiological attributes of fresh-cut cucumbers in controlled atmosphere storage. J. Food Prot. 2020, 83, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ding, Z.; Xie, J. Study of postharvest quality and antioxidant capacity of freshly cut amaranth after blue LED light treatment. Plants 2021, 10, 1614. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. The effect of red and violet light emitting diode (LED) treatments on the postharvest quality and biodiversity of fresh-cut pakchoi (Brassica rapa L. Chinensis). Food Sci. Technol. Int. 2021, 10820132211018892. [Google Scholar] [CrossRef]
- Gutiérrez, D.R.; Chaves, A.R.; Rodríguez, S.D.C. Use of UV-C and gaseous ozone as sanitizing agents for keeping the quality of fresh-cut rocket (Eruca sativa mill). J. Food Process. Preserv. 2017, 41, e12968. [Google Scholar] [CrossRef]
- Sothornvit, R.; Kiatchanapaibul, P. Quality and shelf-life of washed fresh-cut asparagus in modified atmosphere packaging. LWT Food Sci. Technol. 2009, 42, 1484–1490. [Google Scholar] [CrossRef]
- Paillart, M.; van der Vossen, J.; Levin, E.; Lommen, E.; Otma, E.; Snels, J.; Woltering, E. Bacterial population dynamics and sensorial quality loss in modified atmosphere packed fresh-cut iceberg lettuce. Postharvest Biol. Technol. 2017, 124, 91–99. [Google Scholar] [CrossRef]
- Bottino, A.; Degl’Innocenti, E.; Guidi, L.; Graziani, G.; Fogliano, V. Bioactive compounds during storage of fresh-cut spinach: The role of endogenous ascorbic acid in the improvement of product quality. J. Agric. Food Chem. 2009, 57, 2925–2931. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef]
- Qiao, L.; Gao, M.; Zheng, J.; Zhang, J.; Lu, L.; Liu, X. Novel browning alleviation technology for fresh-cut products: Preservation effect of the combination of Sonchus oleraceus L. extract and ultrasound in fresh-cut potatoes. Food Chem. 2021, 348, 129132. [Google Scholar] [CrossRef] [PubMed]
- Guner, S.; Topalcengiz, Z. Effect of pulsed ultraviolet light on natural microbial load and antioxidant properties of fresh blueberries. Turk. J. Agric. Food Sci. Technol. 2018, 6, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yi, P.; Li, C.; Xin, M.; Sun, J.; He, X.; Sheng, J.; Zhou, Z.; Zheng, F.; Li, J. Influence of polysaccharide-based edible coatings on enzymatic browning and oxidative senescence of fresh-cut lettuce. Food Sci. Nutr. 2021, 9, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-L.; Zhao, Y.-T.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Su, X.-G.; Tao, N.-G.; Lakshmanan, P.; Chen, J.-Y. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, G.; Bugatti, V.; Gorrasi, G. Active packaging based on cellulose trays coated with layered double hydroxide as nano-carrier of parahydroxybenzoate: Application to fresh-cut iceberg lettuce. Packag. Technol. Sci. 2021, 34, 353–360. [Google Scholar] [CrossRef]
- Villalobos, M.; Serradilla, M.; Martín, A.; Aranda, E.; López-Corrales, M.; Córdoba, M. Influence of modified atmosphere packaging (MAP) on aroma quality of figs (Ficus carica L.). Postharvest Biol. Technol. 2018, 136, 145–151. [Google Scholar] [CrossRef]
- Dite Hunjek, D.; Pranjić, T.; Repajić, M.; Levaj, B. Fresh-cut potato quality and sensory: Effect of cultivar, age, processing, and cooking during storage. J. Food Sci. 2020, 85, 2296–2309. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Christopoulos, M.V.; Tsantili, E. Short-term treatments with high CO2 and low O2 concentrations on quality of fresh goji berries (Lycium barbarum L.) during cold storage. J. Sci. Food Agric. 2017, 97, 5194–5201. [Google Scholar] [CrossRef]
- Song, Y.; Kim, H.K.; Yam, K.L. Respiration rate of blueberry in modified atmosphere at various temperatures. J. Am. Soc. Hortic. Sci. 1992, 117, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-T.; Zhang, M.; Devahastin, S.; Guo, Z. Effect of combined ultrasonication and modified atmosphere packaging on storage quality of pakchoi (Brassica chinensis L.). Food Bioprocess Technol. 2019, 12, 1573–1583. [Google Scholar] [CrossRef]
- Manolopoulou, H.; Lambrinos, G.; Xanthopoulos, G. Active modified atmosphere packaging of fresh-cut bell peppers: Effect on quality indices. J. Food Res. 2012, 1, 148. [Google Scholar] [CrossRef]
- Rodov, V.; Horev, B.; Goldman, G.; Vinokur, Y.; Fishman, S. Model-driven development of microperforated active modified-atmosphere packaging for fresh-cut produce. In Proceedings of the International Conference on Quality Management of Fresh Cut Produce, Bangkok, Thailand, 6–8 August 2007; pp. 83–88. [Google Scholar]
- Darani, S.; Fazel, M.; Keramat, J. Effect of modified atmosphere packaging on some physicochemical properties of spinach (Spinacia oleracea L.) during storage time. Innov. Food Technol. 2014, 1, 69–80. [Google Scholar]
- Amin, S.; Zaki, M.; Shafshak, N.S.; Abo-Sedera, F.; El-Bassiouny, R. Influence of modified atmosphere packaging on microbiological and sensory quality of artichoke sprouts. Eur. Food Res. Technol. 2002, 215, 21–27. [Google Scholar]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.-J.; Opara, U.L. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences—A review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Gunes, G.; Watkins, C.B.; Hotchkiss, J.H. Physiological responses of fresh-cut apple slices under high CO2 and low O2 partial pressures. Postharvest Biol. Technol. 2001, 22, 197–204. [Google Scholar] [CrossRef]
- Chandra, D.; Lee, J.S.; Hong, Y.P.; Park, M.H.; Choi, A.J.; Kim, J.G. Short-term application of CO2 gas: Effects on physicochemical, microbial, and sensory qualities of “Charlotte” strawberry during storage. J. Food Saf. 2019, 39, e12597. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Irazoqui, M.; Romero, M.; Paulsen, E.; Barrios, S.; Pérez, N.; Faccio, R.; Lema, P. Effect of power ultrasound on quality of fresh-cut lettuce (cv. Vera) packaged in passive modified atmosphere. Food Bioprod. Process. 2019, 117, 138–148. [Google Scholar] [CrossRef]
- De Sousa, J.P.; de Araújo Torres, R.; de Azerêdo, G.A.; Figueiredo, R.C.B.Q.; da Silva Vasconcelos, M.A.; de Souza, E.L. Carvacrol and 1, 8-cineole alone or in combination at sublethal concentrations induce changes in the cell morphology and membrane permeability of Pseudomonas fluorescens in a vegetable-based broth. Int. J. Food Microbiol. 2012, 158, 9–13. [Google Scholar] [CrossRef] [PubMed]
- David, B.V.; Chandrasehar, G.; Selvam, P.N. Pseudomonas fluorescens: A plant-growth-promoting rhizobacterium (PGPR) with potential role in biocontrol of pests of crops. In Crop Improvement Through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–243. [Google Scholar]
- Couvert, O.; Guégan, S.; Hézard, B.; Huchet, V.; Lintz, A.; Thuault, D.; Stahl, V. Modeling carbon dioxide effect in a controlled atmosphere and its interactions with temperature and pH on the growth of L. monocytogenes and P. fluorescens. Food Microbiol. 2017, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.H.; Júnior, L.C.C.; Ferraudo, A.S.; Durigan, J.F. Quality of guava (Psidium guajava L. cv. Pedro Sato) fruit stored in low-O2 controlled atmospheres is negatively affected by increasing levels of CO2. Postharvest Biol. Technol. 2016, 111, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, A.-G.; Kerckhof, F.-M.; Drif, Y.R.; Vanderroost, M.; Boon, N.; Ragaert, P.; De Meulenaer, B.; Devlieghere, F. Characterization of spoilage markers in modified atmosphere packaged iceberg lettuce. Int. J. Food Microbiol. 2018, 279, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Banda, K.; Caleb, O.J.; Jacobs, K.; Opara, U.L. Effect of active-modified atmosphere packaging on the respiration rate and quality of pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2015, 109, 97–105. [Google Scholar] [CrossRef]
- Baniasadi, F.; Saffari, V.R.; Moud, A.A.M. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Hortic. 2018, 234, 312–317. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Fan, J.; Liu, H.; Zhou, J.; Chang, X. iTRAQ proteomics reveals changes in the lettuce (Lactuca sativa L. Grand Rapid) proteome related to colour and senescence under modified atmosphere packaging. J. Sci. Food Agric. 2019, 99, 1908–1918. [Google Scholar] [CrossRef]
- Mayta, M.L.; Lodeyro, A.F.; Guiamet, J.J.; Tognetti, V.B.; Melzer, M.; Hajirezaei, M.R.; Carrillo, N. Expression of a plastid-targeted flavodoxin decreases chloroplast reactive oxygen species accumulation and delays senescence in aging tobacco leaves. Front. Plant Sci. 2018, 9, 1039. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.; Li, D.; Xiang, C.; Jiang, Z.; Wang, J. The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence. Food Chem. 2019, 301, 125224. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Mei, J.; Xie, J. Effects of different carbon dioxide-modified atmosphere packaging and low-temperature storage at 13 °C on the quality and metabolism in mango (Mangifera indica L.). Agriculture 2021, 11, 636. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Buet, A.; Grozeff, G.G.; Galatro, A.; Simontacchi, M. Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Springer: New York, NY, USA, 2017; pp. 177–200. [Google Scholar]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Lu, S.-M.; Kong, F.-C. Effects of low oxygen-modified atmosphere packaging on browning and lignification of peeled bamboo shoots. J. Plant Physiol. Mol. Biol. 2004, 30, 387–392. [Google Scholar]
- Deuchande, T.; Carvalho, S.M.; Giné-Bordonaba, J.; Vasconcelos, M.W.; Larrigaudière, C. Transcriptional and biochemical regulation of internal browning disorder in ‘Rocha’pear as affected by O2 and CO2 concentrations. Postharvest Biol. Technol. 2017, 132, 15–22. [Google Scholar] [CrossRef]
- Khan, M.R.; Chonhenchob, V. Quality changes of longan fruits during storage and shelf life extension. In Asian Berries; CRC Press: Boca Raton, FL, USA, 2020; pp. 173–188. [Google Scholar]
- Serrano, M.; Martinez-Romero, D.; Guillen, F.; Castillo, S.; Valero, D. Maintenance of broccoli quality and functional properties during cold storage as affected by modified atmosphere packaging. Postharvest Biol. Technol. 2006, 39, 61–68. [Google Scholar] [CrossRef]
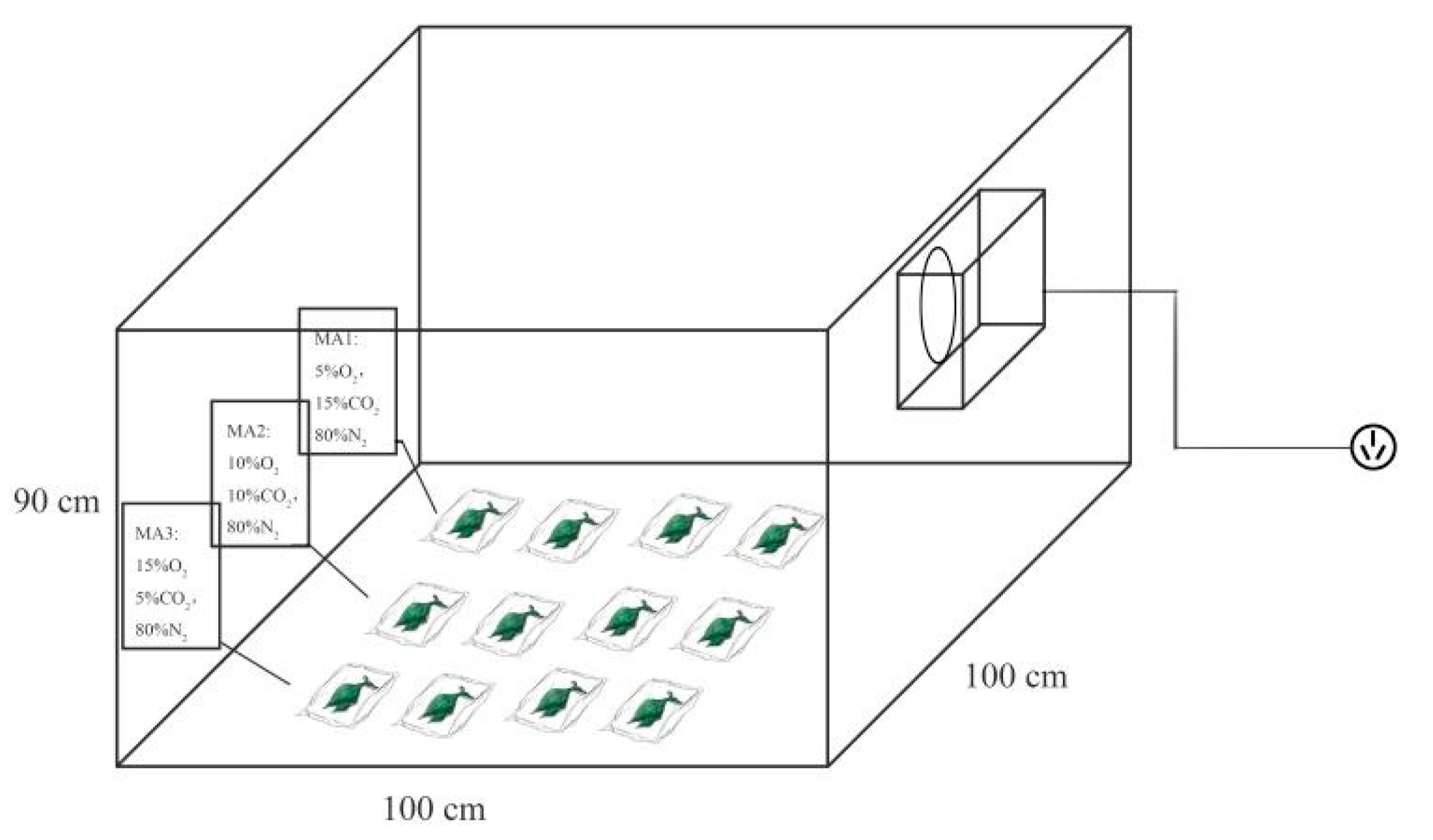






| Group | O2 (%) | CO2 (%) | N2 (%) |
|---|---|---|---|
| CK (air) | 21 | 1 | 78 |
| MA1 | 5 | 15 | 80 |
| MA2 | 10 | 10 | 80 |
| MA3 | 15 | 5 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Ding, Z.; Xie, J. Modified Atmospheric Packaging of Fresh-Cut Amaranth (Amaranthus tricolor L.) for Extending Shelf Life. Agriculture 2021, 11, 1016. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11101016
Jin S, Ding Z, Xie J. Modified Atmospheric Packaging of Fresh-Cut Amaranth (Amaranthus tricolor L.) for Extending Shelf Life. Agriculture. 2021; 11(10):1016. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11101016
Chicago/Turabian StyleJin, Siyuan, Zhaoyang Ding, and Jing Xie. 2021. "Modified Atmospheric Packaging of Fresh-Cut Amaranth (Amaranthus tricolor L.) for Extending Shelf Life" Agriculture 11, no. 10: 1016. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11101016






