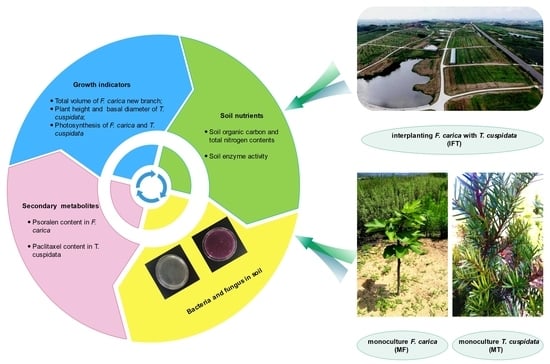
Enhancement of Interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on Growth of Two Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Experiment and Plant Materials
2.2. Experimental Design and Treatments
2.3. Determination of Plant Biomass
2.4. Determination of Plant Photosynthesis
2.5. Soil Sampling and Soil Chemical Analysis
2.5.1. Soil Sampling
2.5.2. Determination of Soil Organic Carbon and Total Nitrogen
2.5.3. Determination of Soil Microbial Community
2.5.4. Dynamic Changes of Soil Enzyme Activities
2.6. Determination of Secondary Metabolites Content
2.6.1. Determination of Psoralen in F. carica
2.6.2. Determination of Paclitaxel in T. cuspidata
2.7. Statistical Analysis
3. Results
3.1. Variation of Plant Biomass in Different Planting Patterns
3.1.1. Variation of F. carica Growth in Different Planting Patterns
3.1.2. Variation of T. cuspidata Growth in Different Planting Patterns
3.2. Variation of Plant Photosynthesis in Different Planting Patterns
3.2.1. Variation of F. carica Photosynthesis in Different Planting Patterns
3.2.2. Variation of T. cuspidata Photosynthesis in Different Planting Patterns
3.3. Variation of Soil Chemical Analysis in Different Planting Patterns
3.3.1. Variation of Soil Organic Carbon and Total Nitrogen in Different Planting Patterns
3.3.2. Variation of Soil Microbial Community in Different Planting Patterns
3.3.3. Variation of Soil Enzyme Activities in Different Planting Patterns
3.4. Variation of Secondary Metabolites Content in Different Planting Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barolo, M.I.; Ruiz Mostacero, N.; López, S.N. Ficus carica L. (Moraceae): An ancient source of food and health. Food Chem. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Li, C.; Yu, M.; Li, S.; Yang, X.; Qiao, B.; Shi, S.; Zhao, C.; Fu, Y. Valorization of Fig (Ficus carica L.) Waste Leaves: HPLC-QTOF-MS/MS-DPPH System for Online Screening and Identification of Antioxidant Compounds. Plants 2021, 10, 2532. [Google Scholar] [CrossRef]
- Sun, R.; Sun, L.; Jia, M. Analysis of psoralen and mineral elements in the leaves of different fig (Ficus carica) cultivars. Acta Hortic. 2017, 1173, 293–296. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Carbonell-Barrachina, Á.A.; Hernández, F. Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J. Funct. Foods 2016, 25, 421–432. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.; Ghoneimy, E.; Abdel-Wahab, A.; Eldeeb, N.; Salem, M.; Salama, E.; Ahmed, T. The therapeutic effects of Ficus carica extract as antioxidant and anticancer agent. S. Afr. J. Bot. 2021, 141, 273–277. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, Y.J.; Xiong, J.; Wang, F.; Xiao, L.J.; Bao, S.Y.; Yu, X.D. Extraction, Purification, and Biological Activities of Flavonoids from Branches and Leaves of Taxus cuspidata S. et Z. Bio. Resources 2021, 16, 2655–2682. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Moon, B.; Baek, K.H. Chemical Composition Analysis and Antibacterial Mode of Action of Taxus Cuspidata Leaf Essential Oil against Foodborne Pathogens. Ital. J. Food Saf. 2014, 34, 9–20. [Google Scholar] [CrossRef]
- Rozendaal, E.V.; Lelyveld, G.P.; Beek, T.V. Screening of the needles of different yew species and cultivars for paclitaxel and related taxoids. Phytochemistry 2000, 53, 383–389. [Google Scholar] [CrossRef]
- Sollen-Norrlin, M.; Ghaley, B.B.; Rintoul, N.L.J. Agroforestry Benefits and Challenges for Adoption in Europe and Beyond. Sustainability 2020, 12, 7001. [Google Scholar] [CrossRef]
- Ilany, T.; Ashton, M.S.; Montagnini, F.; Martinez, C. Using agroforestry to improve soil fertility: Effects of intercropping on Ilex paraguariensis (yerba mate) plantations with Araucaria angustifolia. Agrofor. Syst. 2010, 80, 399–409. [Google Scholar] [CrossRef]
- Pala, N.A. Soil Microbial Characteristics in Sub-Tropical Agro-Ecosystems of North Western Himalaya. Curr. Sci. 2018, 115, 1956–1959. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Gallaher, R.N.; Weldon, C.O.; Boswell, F.C. A semiautomated procedure for total nitrogen in plant and soil samples. Soil Sci. Soc. Am. J. 1976, 40, 887–889. [Google Scholar] [CrossRef]
- Gu, L.; Xu, B.; Liang, Q.; Yin, T. Impact and colonisation ability of on lawn soil microflora. Acta Prataculturae Sin. 2013, 22, 321–326. [Google Scholar]
- Tang, X.; Jiang, J.; Huang, Z.; Wu, H.; Wang, J.; He, L.; Xiong, F.; Zhong, R.; Liu, J.; Han, Z. Sugarcane/peanut intercropping system improves the soil quality and increases the abundance of beneficial microbes. J. Basic Microbiol. 2021, 61, 165–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Ma, X.; Fang, J.; Fan, T.; Wu, F.; An, L.; Feng, H. Microcalorimetric study of the effects of long-term fertilization on soil microbial activity in a wheat field on the Loess Plateau. Ecotoxicology 2014, 23, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhong, R.; Jiang, J.; He, L.; Huang, Z.; Shi, G.; Wu, H.; Liu, J.; Xiong, F.; Han, Z. Cassava/peanut intercropping improves soil quality via rhizospheric microbes increased available nitrogen contents. BMC Biotechnol. 2020, 20, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, S.Y.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Cordero, I.; Snell, H.; Bardgett, R.D. High throughput method for measuring urease activity in soil. Soil Biol. Biochem. 2019, 134, 72–77. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Xu, B.; Yagoub, A.E.G.A.; Mustapha, A.T.; Zhou, C. Effect of intensive pulsed light on the activity, structure, physico-chemical properties and surface topography of polyphenol oxidase from mushroom. Innov. Food Sci. Emerg. Technol. 2021, 72, 102741. [Google Scholar] [CrossRef]
- Wyk, D.A.B.v.; Adeleke, R.; Rhode, O.H.J.; Bezuidenhout, C.C.; Mienie, C. Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt-maize cultivation under field conditions in North West Province of South Africa. J. Basic Microbiol. 2017, 57, 781–792. [Google Scholar] [PubMed]
- Friedel, J.K.; Mölter, K.; Fischer, W.R. Comparison and improvement of methods for determining soil dehydrogenase activity by using triphenyltetrazolium chloride and iodonitrotetrazolium chloride. Biol. Fertil. Soils 1994, 18, 291–296. [Google Scholar] [CrossRef]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal. Biochem. 1997, 245, 55–60. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Lorestani, M.A.; Ansari, M. Extraction and determination of biomarkers in Ficus carica L. leaves from various species and different cultivars by HPLC. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2831–2844. [Google Scholar] [CrossRef]
- Gill, H.; Vasundhara, M. Isolation of taxol producing endophytic fungus Alternaria brassicicola from non-Taxus medicinal plant Terminalia arjuna. World J. Microbiol. Biotechnol. 2019, 35, 74. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yamazaki, H.; Inamoto, K. Effects of interplanting on fruit yield and dry matter production in greenhouse-grown tomato by integratingtwo different crop periods. JARQ Jpn. Agr. Res. Q. 2019, 53, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, R.; Wang, J.; Di, L.; Sikdar, A. The Effects of Leaf Extracts of Four Tree Species on Amygdalus pedunculata Seedlings Growth. Front Plant Sci. 2021, 11, 587579. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Singh, T. Effect of Intercropping Systems and Different Levels of Nutrients on Dry Matter Accumulation and Physiological Growth Parameters of Bed Planted Wheat (Triricum aestivum L.). Indian. J. Sci. Technol. 2015, 8, 11. [Google Scholar] [CrossRef]
- Marconi, L.; Armengot, L. Complex agroforestry systems against biotic homogenization: The case of plants in the herbaceous stratum of cocoa production systems. Agric. Ecosyst. Environ. 2020, 287, 106664. [Google Scholar] [CrossRef]
- Szw, A.; Fsc, A.; Xfh, B.; Yang, Z.A.; Xmf, A. Urbanization aggravates imbalances in the active C, N and P pools of terrestrial ecosystems—ScienceDirect. Glob. Ecol. Conserv. 2020, 21, e00831. [Google Scholar]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Tillage Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Jiang, F.; Drohan, P.J.; Cibin, R.; Preisendanz, H.E.; Veith, T.L. Reallocating crop rotation patterns improves water quality and maintains crop yield. Agric. Syst. 2021, 187, 103015. [Google Scholar] [CrossRef]
- Jensen, E.S. Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 1996, 182, 25–38. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Chen, X. Rhizosphere Processes and Management for Improving Nutrient Use Efficiency and Crop Productivity. Adv. Agron. 2010, 107, 1–32. [Google Scholar]
- Shen, J.; Li, C.; Mi, G.; Li, L.; Yuan, L.; Jiang, R.; Zhang, F. Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J. Exp. Bot. 2013, 64, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, J.; Ritz, K. Plant: Soil interactions in temperate multi-cropping production systems. Plant Soil 2014, 376, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Z.; Arafat, Y.; Lin, W.; Jiang, Y.; Weng, B.; Lin, W.X. Characterizing rhizosphere microbial communities in long-term monoculture tea orchards by fatty acid profiles and substrate utilization—ScienceDirect. Eur. J. Soil Biol. 2017, 81, 48–54. [Google Scholar] [CrossRef]
- Langenberger, G.; Cadisch, G.; Martin, K.; Min, S.; Waibel, H. Rubber intercropping: A viable concept for the 21st century? Agrofor. Syst. 2017, 91, 577–596. [Google Scholar] [CrossRef]
- Qin, X.; Wei, C.; Li, J.; Chen, Y.; Chen, H.S.; Zheng, Y.; Nong, Y.; Liao, C.; Chen, X.; Luo, Y.; et al. Changes in Soil Microbial Community Structure and Functional Diversity in the Rhizosphere Surrounding Tea and Soybean. J. Agri. Sci. Sri Lanka 2017, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, P.E.; Gui, H.; Xu, J.; Zhang, C.; Barrios, E.; Hyde, K.D. Alder trees enhance crop productivity and soil microbial biomass in tea plantations. Appl. Soil Ecol. 2015, 96, 25–32. [Google Scholar] [CrossRef]
- Schandry, N.; Becker, C. Allelopathic Plants: Models for Studying Plant–Interkingdom Interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wu, X.; Nie, C.; Wu, T.; Dai, W.; Liu, H.; Yang, R. Analysis of unculturable bacterial communities in tea orchard soils based on nested PCR-DGGE. World J. Microbiol. Biotechnol. 2012, 28, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rossi, F.; Deng, S.; Liu, Y.; Wang, G.; Adessi, A.; Philippis, R.D. Macromolecular and chemical features of the excreted extracellular polysaccharides in induced biological soil crusts of different ages. Soil Biol. Biochem. 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, J.; Zheng, X.; Zhang, J.; Zhou, P.; Song, X.; Song, W.; Wang, S. Wheat straw and biochar effect on soil carbon fractions, enzyme activities, and nutrients in a tobacco field. Can. J. Soil Sci. 2021, 101, 1–12. [Google Scholar] [CrossRef]
- Rashid, M.; Tigabu, M.; Chen, H.; Farooq, T.H.; Wu, P. Calcium-mediated adaptive responses to low phosphorus stress in Chinese fir. Trees 2020, 34, 825–834. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic Activity of Loess Soil in Organic and Conventional Farming Systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Jan, M.T.; Roberts, P.; Tonheim, S.K.; Jones, D.L. Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils. Soil Biol. Biochem. 2009, 41, 2272–2282. [Google Scholar] [CrossRef]
- Roohi, M.; Arif, M.S.; Yasmeen, T.; Riaz, M.; Bragazza, L. Effects of cropping system and fertilization regime on soil phosphorous are mediated by rhizosphere-microbial processes in a semi-arid agroecosystem. J. Environ. Manag. 2020, 271, 111033. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Gao, S.; Wang, P.; Shang, S. Impacts of simulated nitrogen deposition on soil enzyme activity in a northern temperate forest ecosystem depend on the form and level of added nitrogen. Eur. J. Soil Biol. 2021, 103, 103287. [Google Scholar] [CrossRef]
- Uren, N.C. Types, Amounts, and Possible Functions of Compounds Released into the Rhizosphere by Soil-Grown Plants. Rhizosph. Biochem. Org. Subst. Soil Plant Interface 2007, 2, 1–21. [Google Scholar]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K.; Torabian, S. A short-term study of soil microbial activities and soybean productivity under tillage systems with low soil organic matter. Appl. Soil Ecol. 2021, 168, 104122. [Google Scholar] [CrossRef]
- Wen, F.; Vanetten, H.D.; Tsaprailis, G.; Hawes, M. Extracellular proteins in pea root tip and border cell exudates. Plant Physiol. 2007, 143, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobley, H.; Hu, L.T.; Foxal, P.A. Helicobacter pylori Urease: Properties and Role in Pathogenesis. Scand. J. Gastroenterol. 2009, 26, 39–46. [Google Scholar] [CrossRef]
- Bainard, L.D.; Koch, A.M.; Gordon, A.M.; Klironomos, J.N. Growth response of crops to soil microbial communities from conventional monocropping and tree-based intercropping systems. Plant Soil 2013, 363, 345–356. [Google Scholar] [CrossRef]
- Ngwene, B.; Neugart, S.; Baldermann, S.; Ravi, B.; Schreiner, M. Intercropping Induces Changes in Specific Secondary Metabolite Concentration in Ethiopian Kale (Brassica carinata) and African Nightshade (Solanum scabrum) under Controlled Conditions. Front. Plant Sci. 2017, 8, 1700. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef] [PubMed]








| Month | Planting Patterns | |
|---|---|---|
| Interplanting (cm3) | Monoculture (cm3) | |
| 5 | 45.8 ± 1.1 Ea | 40.5 ± 2.0 Eb |
| 6 | 63.4 ± 1.3 Da | 56.1 ± 1.9 Db |
| 7 | 75.9 ± 1.7 Ca | 67.2 ± 2.1 Cb |
| 8 | 85.7 ± 1.8 Ba | 73.8 ± 1.4 Bb |
| 9 | 96.8 ± 2.1 Aa | 78.4 ± 1.8 Ab |
| Month | Interplanting | Monoculture | ||
|---|---|---|---|---|
| Plant Height (cm) | Growth Rate of Plant Height (%) | Plant Height (cm) | Growth Rate of Plant Height (%) | |
| 4 | 34.1 ± 1.9 Fa | — | 30.2 ± 1.5 Fb | — |
| 5 | 40.9 ± 1.7 Ea | 19.9 ± 1.9 Ea | 34.7 ± 2.4 Eb | 14.9 ± 2.4 Ea |
| 6 | 47.4 ± 2.1 Da | 39.0 ± 1.6 Da | 40.2 ± 1.3 Db | 33.1 ± 0.4 Db |
| 7 | 57.9 ± 1.8 Ca | 69.8 ± 1.8 Ca | 48.7 ± 1.7 Cb | 61.3 ± 0.7 Cb |
| 8 | 68.5 ± 2.4 Ba | 100.8 ± 1.3 Ba | 57.5 ± 1.6 Bb | 90.4 ± 1.8 Bb |
| 9 | 75.6 ± 1.6 Aa | 121.7 ± 1.2 Aa | 61.8 ± 1.3 Ab | 104.6 ± 0.9 Ab |
| Month | Interplanting | Monoculture | ||
|---|---|---|---|---|
| Basal Diameter (mm) | Growth Rate of Basal Diameter (%) | Basal Diameter (mm) | Growth Rate of Basal Diameter (%) | |
| 4 | 14.54 ± 0.11 Fa | — | 14.46 ± 0.17 Fa | — |
| 5 | 15.38 ± 0.32 Ea | 5.8 ± 2.1 Ea | 14.98 ± 0.14 Eb | 3.6 ± 7.8 Eb |
| 6 | 16.89 ± 0.28 Da | 16.2 ± 5.4 Da | 15.71 ± 0.24 Db | 8.6 ± 5.7 Db |
| 7 | 18.53 ± 0.23 Ca | 27.4 ± 2.2 Ca | 16.63 ± 0.33 Cb | 15.0 ± 8.0 Cb |
| 8 | 20.23 ± 0.37 Ba | 39.1 ± 3.2 Ba | 17.35 ± 0.26 Bb | 20.0 ± 4.1 Bb |
| 9 | 21.13 ± 0.25 Aa | 45.3 ± 1.2 Aa | 17.63 ± 0.22 Ab | 21.9 ± 7.3 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, Y.; Li, C.; Li, Q.; Qiao, B.; Shi, S.; Zhao, C. Enhancement of Interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on Growth of Two Plants. Agriculture 2021, 11, 1276. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11121276
Yang X, Li Y, Li C, Li Q, Qiao B, Shi S, Zhao C. Enhancement of Interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on Growth of Two Plants. Agriculture. 2021; 11(12):1276. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11121276
Chicago/Turabian StyleYang, Xue, Yuzheng Li, Chunying Li, Qianqian Li, Bin Qiao, Sen Shi, and Chunjian Zhao. 2021. "Enhancement of Interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on Growth of Two Plants" Agriculture 11, no. 12: 1276. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11121276