Magnesium Supplementation Alters Leaf Metabolic Pathways for Higher Flavor Quality of Oolong Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tea Cultivation and Sampling
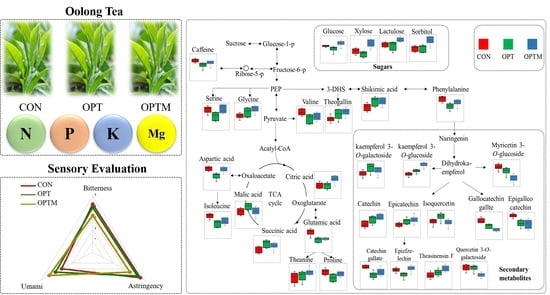
2.2. Sensory Evaluation of Oolong Tea
2.3. Total Polyphenols and Amino Acids Content Analysis
2.4. Chemicals and Reagents
2.5. Non-Targeted Metabolomics Analysis
2.6. Data Processing and Multivariate Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Tea Yield and Quality Analysis
3.2. Identification of Differentail Metabolites between CON, OPT, and OPTM Samples
3.3. Metabolic Pathways and Compositional Differences between CON, OPT, and OPTM Treatments
3.3.1. Sugars
3.3.2. Amino Acids
3.3.3. Flavan-3-ols
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Lv, S.; Wu, Y.; Gao, X.; Li, J.; Zhang, W.; Meng, Q. Oolong tea made from tea plants from different locations in Yunnan and Fujian, China showed similar aroma but different taste characteristics. SpringerPlus 2016, 5, 576. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.-H.; Park, C.Y.; Kim, E.-H.; Park, J.S.; Hong, Y.-S. Diverse metabolite variations in tea (Camellia sinensis L.) leaves grown under various shade conditions revisited: A metabolomics study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.Q.; Granato, D.; Xu, Y.Q.; Ho, C.T. Association between chemistry and taste of tea: A review. Trends Food Sci. Tech. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Fei, Q.; Gao, Y.; Zhang, X.; Sun, Y.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X. Effects of Oolong tea polyphenols, EGCG, and EGCG3″Me on pancreatic alpha-amylase activity in vitro. J. Agric. Food Chem. 2014, 623, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Rumpler, W.; Seale, J.; Clevidence, B.; Judd, J.; Wiley, E.; Yamamoto, S.; Komatsu, T.; Sawaki, T.; Ishikura, Y.; Hosoda, K. Oolong tea increases metabolic rate and fat oxidation in men. J. Nutr. 2001, 131, 2848–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, R.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Ng, K.W.; Cao, Z.J.; Chen, H.B.; Zhao, Z.Z.; Zhu, L.; Yi, T. Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. 2018, 58, 2957–2980. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, Y.L.; Zheng, N.; Li, Y.; Ma, Q.X.; Xiao, H.; Zhou, X.; Xu, X.P.; Jiang, T.M.; He, P.; et al. Temporal variation in nutrient requirements of tea (Camellia sinensis) in China based on QUEFTS analysis. Sci. Rep. UK 2020, 10, 1745. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.L.; Xu, B.; Han, Y.T.; Wang, J.; Wang, X.; Liu, L.L.; Liu, W.X.; Wan, S.Q.; Tan, H.; Liu, Y.Z.; et al. Synthetic nitrogen fertilizers alter the soil chemistry, production and quality of tea. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 10. [Google Scholar] [CrossRef]
- Ruan, L.; Wei, K.; Wang, L.Y.; Cheng, H.; Wu, L.Y.; Li, H.L. Characteristics of free amino acids (the quality chemical components of tea) under spatial heterogeneity of different nitrogen forms in tea (Camellia sinensis) plants. Molecules 2019, 24, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.L.; Zhang, H.Y.; Chen, X.P.; Zhang, C.C.; Ma, W.Q.; Huang, C.D.; Zhang, W.F.; Mi, G.H.; Miao, Y.X.; Li, X.L.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Cui, Z.L.; Fan, M.S.; Zhang, W.F.; Chen, X.P.; Jiang, R.F. Integrated soil-crop system management: Reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. J. Environ. Q. 2011, 40, 1051–1057. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, K.L.; Wang, X.L.; Wang, D.H.; Knudsen, M.T. Carbon footprint and primary energy demand of organic tea in China using a life cycle assessment approach. J. Clean. Prod. 2019, 233, 782–792. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; Zhou, X.B.; He, C.N.; Zhang, F.S. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Liu, X.J.; Hao, T.X.; Zeng, M.F.; Shen, J.B.; Zhang, F.S.; de Vries, W. Cropland acidification increases risk of yield losses and food insecurity in China. Environ. Pollut. 2020, 256, 113145. [Google Scholar] [CrossRef]
- Ui Haq, S.; Boz, I.; Shahbaz, P.; Yildirim, C. Evaluating eco-efficiency and optimal levels of fertilizer use based on the social cost and social benefits in tea production. Environ. Sci. Pollut. Res. 2020, 27, 33008–33019. [Google Scholar] [CrossRef]
- Jayaganesh, S.; Venkatesan, S. Impact of magnesium sulphate on biochemical and quality constituents of black tea. Am. J. Food Technol. 2010, 5, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant. Soil. 2013, 368, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant-soil continuum. Crop. Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Dominguez-Perles, R.; Moreno, D.A.; Muries, B.; Alcaraz-Lopez, C.; Bastias, E.; Garcia-Viguera, C.; Carvajal, M. Minerals in plant food: Effect of agricultural practices and role in human health. A review. Agron Sustain. Dev. 2010, 30, 295–309. [Google Scholar] [CrossRef]
- Guo, W.L.; Nazim, H.; Liang, Z.S.; Yang, D.F. Magnesium deficiency in plants: An urgent problem. Crop. J. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.Y.; Ma, L.F.; Yang, Y.J. Magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food Agric. 2012, 92, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ul Hassan, M.; Nadeem, F.; Wu, L.Q.; Zhang, F.S.; Li, X.X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.Y.; Wu, X.; Ye, Y.; Hardter, R. Effect of potassium, magnesium and sulphur applied in different forms of fertilisers on free amino acid content in leaves of tea (Camellia sinensis L.). J. Sci. Food Agric. 1998, 76, 389–396. [Google Scholar] [CrossRef]
- Ruan, J.Y.; Wu, X.; Hardter, R. Effects of potassium and magnesium nutrition on the quality components of different types of tea. J. Sci. Food Agric. 1999, 79, 47–52. [Google Scholar] [CrossRef]
- Xu, J.L.; Shin, J.S.; Park, S.K.; Kang, S.; Jeong, S.C.; Moon, J.K.; Choi, Y. Differences in the metabolic profiles and antioxidant activities of wild and cultivated black soybeans evaluated by correlation analysis. Food Res. Int. 2017, 100, 166–174. [Google Scholar] [CrossRef]
- Huang, Z. Magnesium Fertilizer Efficiency Evaluation for Tieguanyin (Camellia sinensis (L.) O. Kuntze) and Special Magnesium Fertilizer Applicated Assessment. Master’s Thesis, Fujian Agriculture and Forestry University, Fujian, Beijing, April 2020. (In Chinese). [Google Scholar]
- Huang, Z.; Yin, J.; Huang, Y.; Su, D.; Wu, L. Magnesium fertilizer efficiency and its relation to the nutrient absorption and utilization of tea plant in oolong tea producing area. J. Southern Agric. 2020, 51, 2120–2129. [Google Scholar] [CrossRef]
- Fraser, K.; Lane, G.A.; Otter, D.E.; Harrison, S.J.; Quek, S.Y.; Hemar, Y.; Rasmussen, S. Non-targeted analysis by LC-MS of major metabolite changes during the oolong tea manufacturing in New Zealand. Food Chem. 2014, 151, 394–403. [Google Scholar] [CrossRef] [PubMed]
- National Standard of the People’s Republic of China, GB/T 8313. Determination of Total Polyphenols and Catechins Content in Tea; Standardization Administration of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- National Standard of the People’s Republic of China, GB/T 8314. Determination of Free Amino Acids Content in Tea; Standardization Administration of the People’s Republic of China: Beijing, China, 2013. (In Chinese)
- Hyeon, H.; Xu, J.L.; Kim, J.K.; Choi, Y. Comparative metabolic profiling of cultivated and wild black soybeans reveals distinct metabolic alterations associated with their domestication. Food Res. Int. 2020, 134, 109290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Shi, Y.Z.; Ma, L.F.; Yi, X.Y.; Ruan, J.Y. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. PLoS ONE 2014, 9, 112572. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.F.; Ruan, J.Y. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 47–80. [Google Scholar] [CrossRef]
- Mehra, A.; Baker, C.L. Leaching and bioavailability of aluminium, copper and manganese from tea (Camellia sinensis). Food Chem. 2007, 100, 1456–1463. [Google Scholar] [CrossRef]
- Liang, Y.L.; Lu, J.L.; Zhang, L.Y.; Wu, S.; Wu, Y. Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusions. Food Chem. 2003, 80, 283–290. [Google Scholar] [CrossRef]
- Lin, X.H.; Sun, W.D. Recent developments in vibrational spectroscopic techniques for tea quality and safety analyses. Trends Food Sci. Tech. 2020, 104, 163–176. [Google Scholar] [CrossRef]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stitt, M. Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant. J. 2006, 46, 533–548. [Google Scholar] [CrossRef]
- Scharbert, S.; Hofmann, T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Zhou, Y.; Fu, X.M.; Mei, X.; Cheng, S.H.; Gui, J.D.; Dong, F.; Tang, J.C.; Ma, S.Z.; Yang, Z.Y. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017, 237, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Li, H.P.; Tai, Y.L.; Dong, C.X.; Cheng, X.M.; Xia, E.H.; Chen, Z.P.; Li, F.; Wan, X.C.; Zhang, Z.L. Transcriptional regulation of amino acid metabolism in response to nitrogen deficiency and nitrogen forms in tea plant root (Camellia sinensis L.). Sci Rep. UK 2020, 10, 6868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okemwa, E.K.; Silvanuss, K.K. Effects of different fertilizer rates on total polyphenols and catechins of selected clones of green tea (Camellia sinensis L. [O] Kuntze). World J. Appl. Chem. 2020, 5, 13–19. [Google Scholar] [CrossRef]
- Dong, F.; Hu, J.H.; Shi, Y.Z.; Liu, M.Y.; Zhang, Q.F.; Ruan, J.Y. Effects of nitrogen supply on flavonol glycoside biosynthesis and accumulation in tea leaves (Camellia sinensis). Plant. Physiol. Bioch. 2019, 138, 48–57. [Google Scholar] [CrossRef]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.; Wright, J.S.; Hall, J.W.; Gong , P.; Ni, S.; Qiao, S.; Huang, G.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef]
- Lin, Z.H.; Qi, Y.P.; Chen, R.B.; Zhang, F.Z.; Chen, L.S. Effects of phosphorus supply on the quality of green tea. Food Chem. 2012, 130, 908–914. [Google Scholar] [CrossRef]
- Liu, P.P.; Yin, J.F.; Chen, G.S.; Wang, F.; Xu, Y.Q. Flavor characteristics and chemical compositions of oolong tea processed using different semi-fermentation times. J. Food Sci. Techchnol. 2018, 55, 1185–1195. [Google Scholar] [CrossRef]
- Dong, C.X.; Li, F.; Yang, T.Y.; Feng, L.; Zhang, S.P.; Li, F.D.; Li, W.H.; Xu, G.H.; Bao, S.L.; Wan, X.C.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant. J. 2020, 101, 57–70. [Google Scholar] [CrossRef]
- Deng, W.W.; Ogita, S.; Ashihara, H. Distribution and biosynthesis of theanine in Theaceae plants. Plant. Physiol. Bioch. 2010, 48, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Haerdter, R.; Gerendas, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant. Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wu, L.; Liao, H. Comprehensive analysis revealed the close relationship between N/P/K status and secondary metabolites in tea leaves. ACS Omega 2019, 4, 176–184. [Google Scholar] [CrossRef] [Green Version]




| Treatment | TP (mg/g DW) | FAA (mg/g DW) | Ratio of TP to FAA |
|---|---|---|---|
| CON | 158.3 ± 9.3 b | 26.5 ± 2.3 b | 6.0 ± 0.3 a |
| OPT | 165.6 ± 10.3 a | 26.3 ± 1.7 b | 6.3 ± 0.4 a |
| OPTM | 158.7 ± 7.6 b | 28.3 ± 2.4 a | 5.6 ± 0.3 b |
| NO. | Rt (min) | Theoretical Mass (m/z) | Accurate Mass (m/z) | Error (ppm) | ID | MS2 a Fragments | VIP b | p Value c |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.61 | 307.0813 | 307.0804 | −2.9 | Epigallcocatechin d | 289, 153, 139 | 1.30 | 4.85 × 10−4 |
| 2 | 2.86 | 291.0863 | 291.0856 | −2.4 | Catechin d | 139, 123, 95 | 1.20 | 2.30 × 10−2 |
| 3 | 3.19 | 195.0877 | 195.0871 | −3.1 | Caffeine d | 138, 110, 69 | 1.11 | 2.56 × 10−2 |
| 4 | 3.44 | 291.0863 | 291.0856 | −2.4 | Epichatechin d | 207, 139, 123 | 1.10 | 3.30 × 10−2 |
| 5 | 3.45 | 459.0922 | 459.0914 | −1.7 | Gallocatechingallte d | 289, 181, 153 | 1.44 | 1.30 × 10−5 |
| 6 | 3.49 | 899.1678 | 899.1688 | 1.1 | Theasinensin F d | 425, 287,153 | 1.36 | 1.95 × 10−4 |
| 7 | 3.89 | 275.0914 | 275.0911 | −1.1 | Epizfzelechin d | 191, 139, 107 | 1.36 | 2.17 × 10−3 |
| 8 | 3.98 | 481.0977 | 481.0955 | −4.5 | Myricetin 3-O-glucoside d | 319 | 1.37 | 2.81 × 10−4 |
| 9 | 4.20 | 443.0973 | 443.0959 | −3.1 | Catechin gallate d | 273, 153, 139 | 1.35 | 5.68 × 10−4 |
| 10 | 4.24 | 773.2135 | 773.2129 | 0.78 | Quercetin 3-O-glucosyl-rutinoside | 611, 465, 303 | 1.68 | 2.31 × 10−3 |
| 11 | 4.42 | 611.1607 | 611.1600 | −1.15 | Rutin d | 465, 303, 85 | 1.43 | 2.30 × 10−3 |
| 12 | 4.48 | 465.1028 | 465.0998 | −6.5 | Quercetin 3-O-galactoside | 303, 165, 91 | 1.38 | 4.12 × 10−4 |
| 13 | 4.56 | 465.3742 | 465.3756 | 3.0 | Isoquercitrin d | 303 | 1.13 | 1.59 × 10−2 |
| 14 | 4.87 | 449.1079 | 449.1053 | −5.8 | Kaempferol 3-O-glucoside | 287, 85 | 1.50 | 3.20 × 10−2 |
| 15 | 5.04 | 449.1079 | 449.1056 | −5.1 | Kaempferol 3-O-galactoside | 287 | 1.02 | 4.30 × 10−2 |
| NO | Rt (min) | ID | MS2 a Fragments | VIP b | p Value c |
|---|---|---|---|---|---|
| Sugars | |||||
| 1 | 15.61 | Xylose d | 217, 117, 74 | 1.49 | 6.70 × 10−5 |
| 2 | 18.10 | Glucose d | 205, 147, 103 | 1.32 | 4.52 × 10−3 |
| 3 | 18.60 | Sorbitol d | 219, 293, 308 | 1.31 | 1.52 × 10−3 |
| 4 | 24.93 | Lactulose d | 204, 131, 147 | 1.13 | 3.20 × 10−3 |
| Amino acids | |||||
| 5 | 8.61 | Valine d | 144, 218, 100 | 1.09 | 2.56 × 10−2 |
| 6 | 9.73 | Isoleucine d | 158, 218, 100 | 1.31 | 1.49 × 10−3 |
| 7 | 10.68 | Serine d | 204, 218, 147 | 1.24 | 9.07 × 10−3 |
| 8 | 11.08 | Proline d | 142, 244, 216 | 1.13 | 1.40 × 10−2 |
| 9 | 12.77 | Aspartic acid d | 232, 100, 147 | 1.43 | 1.00 × 10−4 |
| 10 | 14.15 | Glutamic acid d | 246, 147, 100 | 1.37 | 9.80 × 10−4 |
| 11 | 14.42 | Phenylalanine d | 218, 192, 147 | 1.26 | 3.80 × 10−2 |
| 12 | 14.98 | Theanine d | 160, 273, 128 | 1.03 | 2.60 × 10−2 |
| Organic acids | |||||
| 13 | 8.69 | Oxalic acid d | 147, 218, 190 | 1.53 | 3.30 × 10−5 |
| 14 | 11.30 | Succinic acid d | 147, 247, 73 | 1.10 | 2.10 × 10−2 |
| 15 | 13.57 | Malic acid d | 147, 233, 245 | 1.51 | 1.10 × 10−2 |
| 16 | 17.26 | Shikimic acid d | 204, 147, 267 | 1.05 | 2.40 × 10−2 |
| 17 | 17.36 | Citric acid d | 273, 147, 347 | 1.28 | 3.00 × 10−3 |
| 18 | 15.28 | Tartaric acid | 147, 186, 217 | 1.49 | 5.70 × 10−5 |
| 19 | 8.28 | Saccharic acid | 143, 217, 333 | 1.33 | 7.74 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wu, L.; Tong, B.; Yin, J.; Huang, Z.; Li, W.; Li, X. Magnesium Supplementation Alters Leaf Metabolic Pathways for Higher Flavor Quality of Oolong Tea. Agriculture 2021, 11, 120. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11020120
Xu J, Wu L, Tong B, Yin J, Huang Z, Li W, Li X. Magnesium Supplementation Alters Leaf Metabolic Pathways for Higher Flavor Quality of Oolong Tea. Agriculture. 2021; 11(2):120. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11020120
Chicago/Turabian StyleXu, Jiuliang, Liangquan Wu, Bingxin Tong, Jiaxu Yin, Zican Huang, Wei Li, and Xuexian Li. 2021. "Magnesium Supplementation Alters Leaf Metabolic Pathways for Higher Flavor Quality of Oolong Tea" Agriculture 11, no. 2: 120. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11020120