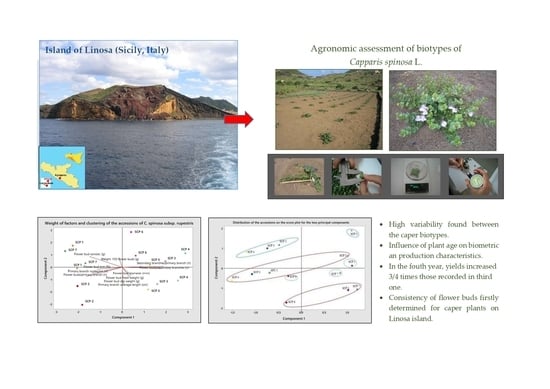
Four-Year Study on the Bio-Agronomic Response of Biotypes of Capparis spinosa L. on the Island of Linosa (Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Cropping Techniques and Plant Material
2.2. Plant Measurement
2.3. Statistical Analysis
3. Results
3.1. Analysis of Rainfall and Air Temperature Trends at the Test Site
3.2. Analysis of Biometric and Production Parameters
3.3. Correlation Matrix
3.4. PCA Analysis
3.5. Phenology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, J.C.; Sytsma, K.J.; Iltis, H.H. Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data. Am. J. Bot. 2002, 89, 1826–1842. [Google Scholar] [CrossRef]
- Jacobs, M. The genus Capparis (Capparaceae) from the Indus to the Pacific. Blumea 1965, 12, 385–541. [Google Scholar]
- Fici, S. Intraspecific variation and evolutionary trends in Capparis spinosa L. (Capparaceae). Plant Syst. Evol. 2001, 228, 123–141. [Google Scholar] [CrossRef]
- Sozzi, O.G. Caper bush: Botany and horticulture. Hortic. Rev. 2001, 27, 125–188. [Google Scholar]
- Rivera, D.; Inocencio, C.; Obón, C.; Alcaraz, F. Review of food and medicinal uses of Capparis L. subgenus Capparis (Capparidaceae). Econ. Bot. 2003, 57, 515–534. [Google Scholar] [CrossRef]
- Bhoyar, M.S.; Mishra, P.G.; Naik, K.P.; Murkute, A.A.; Srivastavar, B.R. Genetic variability studies among natural populations of Capparis spinosa from cold arid desert of trans-Himalayas using DNA markers. Natl. Acad. Sci. Lett. 2012, 35, 505–515. [Google Scholar] [CrossRef]
- Tlili, N.; El-Fallah, W.; Saadadoui, E.; Khaldi, A.H.; Triki, S.; Nasri, N. The caper (Capparis L.): Ethnopharmacology, phyto-chemical and pharmacological properties. Fitoterapia 2011, 82, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V.H. Capparis L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; p. 312. [Google Scholar]
- Gristina, A.S.; Fici, S.; Siragusa, M.; Fontana, I.; Garfì, G.; Carimi, F. Hybridization in Capparis spinosa L.: Molecular and morphological evidence from a Mediterranean island complex. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 733–741. [Google Scholar] [CrossRef]
- Chedraoui, S.; Abi-Rizk, A.; El-Beyrouthy, M.; Chalak, L.; Ouaini, N.; Rajjou, L. Capparis spinosa L. in a systematic review: A xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front. Plant Sci. 2017, 8, 1845. [Google Scholar] [CrossRef] [Green Version]
- Saadaoui, E.; Khaldi, A.; Khouja, M.L.; Mohamed, E.G. Intraspecific variation of Capparis spinosa L. in Tunisia. J. Herbs Spices Med. Plants 2009, 15, 9–15. [Google Scholar] [CrossRef]
- Sozzi, O.G.; Vicente, A.R. Capers and caperberries. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing Limited and CRC Press: Boca Raton, FL, USA, 2006; pp. 230–256. [Google Scholar]
- Aliyazicioglu, R.; Tosun, G.; Eyupoglu, E. Characterisation of volatile compounds by spme and gc-fid/ms of capers (Capparis spinosa L.). Afr. J. Agric. Res. 2015, 10, 2213–2217. [Google Scholar]
- Legua, P.; Martínez, J.; Melgarejo, P.; Hernández, F. Phenological growth stages of caper plant (Capparis spinosa L.) according to the Biologische Bundesanstalt, Bundessortenamt and CHemical scale. Ann. Appl. Biol. 2013, 163, 135–141. [Google Scholar] [CrossRef]
- Romeo, V.; Ziino, M.; Giuffrida, D.; Condurso, C.; Verzera, A. Flavor profile of Capers (Capparis spinosa L.) from the Eolian Archipelago by HS-SPME/GC-MS. Food Chem. 2007, 101, 1272–1278. [Google Scholar] [CrossRef]
- Barbera, G. Il Cappero; Edagricole: Bologna, Italy, 1993. [Google Scholar]
- Lorestani, A.N. Some physical and mechanical properties of caper. J. Agric. Technol. 2012, 8, 1199–1206. [Google Scholar]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Gargano, M.L.; Venturella, G.; La Bella, S. Plant genetic resources and traditional knowledge on medicinal use of wild shrub and herbaceous plant species in the Etna Regional Park (Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 155, 1362–1381. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.N.; Eddouks, M.; Michel, J.B.; Sulpice, T.; Hajji, L. Cardiovascular effect of capparis spinosa aqueous extract. Part III: Antihypertensive effect in spontaneously hypertensive rats. Am. J. Pharmacol. Toxicol. 2007, 2, 111–115. [Google Scholar] [CrossRef]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef]
- Kazemian, M.; Abad, M.; Haeri, M.R.; Ebrahimi, M.; Heidari, R. Anti-diabetic effect of Capparis spinosa L. root extract in diabetic rats. Avicenna J. Phytomed. 2015, 5, 325–332. [Google Scholar]
- Mollica, A.; Zengin, G.; Locatelli, M.; Stefanucci, A.; Mocan, A.; Macedonio, G.; Carradori, S.; Onaolapo, O.; Onaolapo, A.; Adegoke, J.; et al. Anti-diabetic and anti-hyperlipidemic properties of Capparis spinosa L.: In vivo and in vitro evaluation of its nutraceutical potential. J. Funct. Foods 2017, 35, 32–42. [Google Scholar] [CrossRef]
- Lemhadri, A.; Eddouks, M.; Sulpice, T.; Burcelin, R. Anti-hyperglycaemic and anti-obesity effects of Capparis spinosa and Chamaemelum nobile aqueous extracts in HFD mice. Am. J. Pharmacol. Toxicol. 2007, 2, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, D.; Occhiuto, F.; Perri, D.; Puglia, C.; Santagati, N.A.; Pasquale, A.D.; Saija, A.; Bonina, F. Antiallergic and anti-histaminic effect of two extracts of Capparis spinosa L. flowering buds. Phytother. Res. 2005, 19, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jian, R.; Kang, J.; Huang, X.; Li, Y.; Zhuang, C.; Yang, F.; Zhang, L.; Fan, X.; Wu, T.; et al. Anti-inflammatory effects of caper (Capparis spinosa L.) fruit aqueous extract and the isolation of main phytochemicals. J. Agric. Food Chem. 2010, 58, 12717–12721. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Mahboubi, A. Antimicrobial activity of Capparis spinosa as its usages in traditional medicine. Herba Pol. 2014, 60, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Tlili, N.; Khaldi, A.; Triki, S.; Munné-Bosch, S. Phenolic compounds and vitamin antioxidants of caper (Capparis spinosa). Plant Foods Hum. Nutr. 2010, 65, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) priority in Italy: Distribution, ecology, in situ and ex situ conservation and expected actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Yang, T.; Wang, C.; Liu, H.; Chou, G.; Cheng, X.; Wang, Z. A new antioxidant compound from Capparis spinosa. Pharm. Biol. 2010, 48, 589–594. [Google Scholar] [CrossRef]
- Yang, T.; Wang, C.-H.; Chou, G.-X.; Wu, T.; Cheng, X.-M.; Wang, Z.-T. New alkaloids from Capparis spinosa: Structure and X-ray crystallographic analysis. Food Chem. 2010, 123, 705–710. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, D.-B.; He, J.-B.; Guan, K.-Y.; Zhu, H.-J. A novel tetrahydroquinoline acid and a new racemic benzofuranone from Capparis spinosa L., a case study of absolute configuration determination using quantum methods. Tetrahedron 2014, 70, 869–873. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A. Flavonoids of four Cleome and three Capparis species. Biochem. Syst. Ecol. 1997, 25, 161–166. [Google Scholar] [CrossRef]
- Kulisic-Bilusic, T.; Blažević, I.; Dejanović, B.; Miloš, M.; Pifat, G. Evaluation of the antioxidant activity of essential oils from caper (Capparis spinosa) and sea fennel (Crithmum maritimum) by different methods. J. Food Biochem. 2010, 34, 286–302. [Google Scholar] [CrossRef]
- Fu, X.P.; Wu, T.; Abdurahim, M.; Su, Z.; Hou, X.L.; Aisa, H.A.; Wu, H. New spermidine alkaloids from Capparis spinosa roots. Phytochem. Lett. 2008, 1, 59–62. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F. Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Francesca, N.; Barbera, M.; Martorana, A.; Saiano, F.; Gaglio, R.; Aponte, M.; Moschetti, G.; Settanni, L. Optimised method for the analysis of phenolic compounds from caper (Capparis spinosa L.) berries and monitoring of their changes during fermen-tation. Food Chem. 2016, 196, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.; Smith, J.C. Repurposing therapeutics for COVID-19: Supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. ChemRxiv 2020, in press. [Google Scholar]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Haslberger, A.G.; Jacob, U.; Hippe, B.; Karlic, H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: Mini review. Funct. Food Health Dis. 2020, 5, 195–209. [Google Scholar] [CrossRef]
- Bailey, L. The Standard Cyclopedia of Horticulture; Macmillan: New York, NY, USA, 1927; Volume 1, p. 658. [Google Scholar]
- Baccaro, G. Il Cappero: Pianta da Reddito; Universale Edagricole, Edizioni Edagricole: Bologna, Italy, 1987. [Google Scholar]
- Faran, M. Capparis spinosa—The plant on the wall. In Medicinal and Aromatic Plants of the Middle-East (Medicinal and Aromatic Plants of the World); Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 59–65. [Google Scholar]
- Zuo, W.; Ma, M.; Ma, Z.; Gao, R.; Guo, Y.; Jiang, W.; Liu, J.; Tian, L. Study of photosynthetic physiological characteristics of desert plant Capparis spinosa L. J. Shihezi Univ. 2012, 3, 006. [Google Scholar]
- Gan, L.; Zhang, C.; Yin, Y.; Lin, Z.; Huang, Y.; Xiang, J.; Fu, C.; Li, M. Anatomical adaptations of the xerophilous medicinal plant, Capparis spinosa, to drought conditions. Hortic. Environ. Biotechnol. 2013, 54, 156–161. [Google Scholar] [CrossRef]
- Grimalt, M.; Hernández, F.; Legua, P.; Almansa, M.; Amorós, A. Physicochemical composition and antioxidant activity of three Spanish caper (Capparis spinosa L.) fruit cultivars in three stages of development. Sci. Hortic. 2018, 240, 509–515. [Google Scholar] [CrossRef]
- La Mantia, T.; Rühl, J.; Massa, B.; Pipitone, S.; Lo Verde, G.; Bueno, R.S. Vertebrate-mediated seed rain and artificial perches contribute to overcome seed dispersal limitation in a Mediterranean old field. Restor. Ecol. 2019, 27, 1393–1400. [Google Scholar] [CrossRef]
- Barbera, G. Le câprier (Capparis spp.). In Programme de Recherche Agrimed; Guiseppe, B., Ed.; Commission des Communautés Européennes L-2920: Luxembourg, 1991; p. 62. [Google Scholar]
- Fierotti, G. I Suoli della Sicilia: Con Elementi di Genesi, Classificazione, Cartografia e Valutazione dei Suol, 1st ed.; Flaccovio Dario: Palermo, Italy, 1997. [Google Scholar]
- Tuttolomondo, T.; La Bella, S.; Cammalleri, I.; Gaglio, G. Indagine preliminare sul germoplasma di Capparis spinosa subsp. rupestris (Sm.) Nyman dell’isola di Linosa. Ital. J. Agron. 2009, 4, 513–517. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; La Bella, S.; Hornsby, L. Il cappero: Analisi desk sui flussi di commercializzazione in Italia e nei principali Paesi competitors. Ital. J. Agron. 2009, 4, 789–796. [Google Scholar]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; La Bella, S. Agronomic evaluation and chemical characterization of Sicilian Salvia sclarea L. accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- Perrino, E.V.; Perrino, P. Crop wild relatives: Know how past and present to improve future research, conservation and utilization strategies, especially in Italy: A review. Genet. Resour. Crop. Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Landucci, F.; Panella, L.; Lucarini, D.; Gigante, D.; Donnini, D.; Kell, S.; Maxted, N.; Venanzoni, R.; Negri, V. A prioritized inventory of crop wild relatives and wild harvested plants of Italy. Crop. Sci. 2014, 54, 1628–1644. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado. Normas de calidad para el comercio exterior de alcaparras y alcaparrones. BOE 1984, 100, 11394–11397. [Google Scholar]
- Inocencio, C.; Rivera, D.; Obon, C.; Alcaraz, F.; Barrena, J.A. A systematic revision of Capparis section Capparis (Capparaceae). Ann. Mo. Bot. Gard. 2006, 93, 122–149. [Google Scholar] [CrossRef]
- Fici, S.; Gianguzzi, L. Diversity and conservation in wild and cultivated Capparis in Sicily. Bocconea 1997, 7, 437–443. [Google Scholar]
- Aytaç, Z.; Kinaci, G.; Caylan, A. Yield and some morphological characteristics of Caper (Capparis spinosa L.) population cultivated at various slopes in Aegean ecological conditions. Pak. J. Bot. 2009, 41, 591–596. [Google Scholar]
- Barbera, G.; Di Lorenzo, R. La coltura specializzata del cappero nell’ isola di Pantelleria. Inf. Agrar. 1982, XXXVIII, 22113–22117. [Google Scholar]
- Shewfelt, R.L. What is quality? Postharvest Biol. Technol. 1999, 15, 197–200. [Google Scholar] [CrossRef]
- Crisosto, C.H. Stone fruit maturity indices: A descriptive. Postharvest News Inf. 1994, 5, 65–68. [Google Scholar]
- Melgarejo, P.; Legua, P.; Martinez, J.; Martinez-Font, R.; Hernandez, F. Preliminary characterization of sixty one caper clones (Capparis spinosa L.). Acta Hortic. 2009, 818, 155–160. [Google Scholar] [CrossRef]
- Burghardt, L.T.; Edwards, B.R.; Donohue, K. Multiple paths to similar germination behavior in Arabidopsis thaliana. New Phytol. 2015, 209, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Awatef, R.; Hédia, H.; Sonia, H.; Mohamed, B. The use of morphological descriptors to study variability in wild populations of Capparis spinosa L. (Capparaceae) in Tunisia. Afr. J. Ecol. 2012, 51, 47–54. [Google Scholar] [CrossRef]






| Biotype | Leaf Color (Code n.) | Leaf Morphology | Spiny Stipulates | Flower Bud Color (Code Number) * | Bud Morphology |
|---|---|---|---|---|---|
| SCP1 | brown-green (371) | obovate leaves with retuse apices | absent | deep-green (412) | rounded |
| SCP2 | brown-green (371) | obovate leaves with retuse apices | absent | deep-green (412) | rounded |
| SCP3 | brown-green (371) | ovate leaves with marked retuse apices | absent | deep-green (412) | rounded/ pyramidal |
| SCP4 | deep-green (421) | ovate leaves | absent | deep-green (411) | rounded/ pyramidal |
| SCP5 | deep-green (421) | ovate leaves with marked retuse apices | absent | deep-green (412) | rounded/ pyramidal |
| SCP6 | deep-green (426) | ovate leaves with marked retuse apices | absent | deep-green with dark spots (422) | rounded/ pyramidal |
| SCP7 | deep-green (426) | Ovate leaves with marked retuse apices | absent | deep-green with dark spots (423) | rounded |
| Factor | Flower Bud Fresh Weight (g) | Flower Bud Dry Weight (g) | Weight 100 Flower Bud (g) | Flower Bud Dry Matter (%) | Flower Bud Diameter (mm) | Flower Bud Consistency (g) | Primary Branch Average Length (cm) | Primary Branch Nodes cm−1 (n) | Secondary Branch/ Primary Branch (n) | Flower Buds/ Primary Branch (n) | Flower Buds/ Secondary Branch (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biotype (B) | |||||||||||
| SCP1 | 1031.89 ab | 187.24 ab | 20.63 c | 17.79 bc | 7.57 c | 975.47 a | 104.21 d | 0.66 b | 5.77 c | 36.08 b | 15.13 f |
| SCP2 | 1167.61 a | 220.83 a | 22.84 b | 18.64 ab | 8.19 ab | 886.67 d | 139.19 a | 0.70 a | 6.37 c | 46.58 a | 25.51 e |
| SCP3 | 1056.70 ab | 184.57 ab | 22.08 b | 17.23 c | 7.65 c | 855.97 e | 127.24 b | 0.64 bc | 19.07 a | 25.43 c | 47.01 a |
| SCP4 | 1114.43 ab | 160.70 b | 23.40 b | 14.23 d | 8.15 ab | 842.16 f | 117.78 c | 0.61 c | 18.58 a | 24.17 c | 43.72 b |
| SCP5 | 1099.20 ab | 212.70 a | 23.04 b | 19.11 a | 8.49 a | 856.59 e | 117.92 c | 0.65 bc | 18.94 a | 25.00 c | 37.68 d |
| SCP6 | 919.16 bc | 177.44 ab | 28.12 a | 19.02 a | 7.58 c | 922.85 c | 99.74 e | 0.64 bc | 17.37 b | 24.46 c | 41.41 c |
| SCP7 | 745.17 c | 147.97 b | 27.80 a | 19.48 a | 7.97 bc | 942.10 b | 114.77 c | 0.66 b | 6.62 c | 35.94 b | 14.16 f |
| Year (Y) | |||||||||||
| 2007 | 446.00 b | 77.53 b | 24.11 a | 17.46 b | 8.01 a | 898.82 a | 114.13 b | 0.66 a | 12.87 b | 29.67 b | 31.77 a |
| 2008 | 1592.33 a | 291.44 a | 23.86 a | 18.39 a | 7.87 a | 895.99 a | 120.40 a | 0.65 a | 13.62 a | 32.52 a | 32.41 a |
| Y × B | * | * | * | * | * | ** | * | ** | * | * | ** |
| 2008 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characters | FBFW/P | FBDW/P | W100FB | FBDM | FBD | FBC | PBAL | PBN | SBPB | FBPB | FBSB | |
| 2007 | FBFW/P | 0.7002 | −0.8101 * | −0.5108 | 0.3024 | −0.5619 | 0.4757 | 0.6516 | 0.2236 | 0.0232 | 0.3796 | |
| FBDW/P | 0.7830 * | −0.564 | 0.2515 | 0.2249 | −0.2139 | 0.4032 | 0.8684 * | −0.0813 | 0.3368 | 0.0534 | ||
| W100FB | −0.6078 | −0.3776 | 0.4343 | −0.019 | 0.2082 | −0.3884 | −0.5368 | 0.0527 | −0.1581 | −0.0324 | ||
| FBDM | −0.3115 | 0.3465 | 0.3651 | −0.0941 | 0.4765 | −0.1324 | 0.1596 | −0.3932 | 0.3782 | −0.4446 | ||
| FBD | 0.5340 | 0.5728 | −0.1761 | 0.0887 | −0.6163 | 0.4530 | 0.0717 | 0.2176 | 0.0069 | 0.1378 | ||
| FBC | −0.6513 | −0.3213 | 0.1985 | 0.4819 | −0.5068 | −0.5733 | −0.0409 | 0.7607 * | 0.4574 | −0.821 * | ||
| PBAL | 0.6478 | 0.5935 | −0.3399 | −0.059 | 0.5296 | −0.5468 | 0.5314 | −0.028 | 0.3941 | 0.1760 | ||
| PBN | −0.1514 | 0.3560 | 0.1526 | 0.7813 * | 0.2248 | 0.5360 | 0.2839 | −0.4022 | 0.6623 | −0.1507 | ||
| SBPB | 0.2767 | 0.0482 | 0.0363 | −0.3481 | 0.0432 | −0.7105 | −0.1299 | −0.8002 * | −0.9116 ** | 0.9351 ** | ||
| FBPB | 0.1124 | 0.3145 | −0.1459 | 0.3193 | 0.1362 | 0.4115 | 0.5069 | 0.8235 * | −0.8903 ** | −0.7551 * | ||
| FBSB | 0.4199 | 0.1261 | −0.016 | −0.4437 | −0.0361 | −0.7621 * | 0.0469 | −0.7885 * | 0.9491 ** | −0.7396 * | ||
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalues | 4.01 | 2.62 | 1.88 |
| % variance | 36.44 | 23.82 | 17.04 |
| % cumulative variance | 36.44 | 60.26 | 77.30 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Flower bud fresh weight/plant (g) | −0.0170 | 0.7726 | 0.5975 |
| Flower bud dry weight/plant (g) | 0.0781 | 0.7551 | 0.6347 |
| Weight 100 flower buds (g) | 0.0483 | −0.4720 | 0.2717 |
| Flower bud dry matter (%) | 0.5838 | 0.0633 | 0.3437 |
| Flower bud diameter (mm) | −0.1576 | 0.3101 | −0.6735 |
| Flower bud consistency (g) | 0.7826 | −0.4748 | 0.3420 |
| Primary branch average length (cm) | 0.0313 | 0.8279 | −0.4013 |
| Primary branch nodes/cm−1 (n/cm) | 0.7238 | 0.2111 | −0.3868 |
| Second. branches/primary branch (n) | −0.9526 | 0.0376 | 0.1472 |
| Flower buds/primary branch (n) | 0.8570 | 0.3944 | −0.1708 |
| Flower buds/secondary branches (n) | −0.9240 | 0.1302 | 0.0663 |
| Year | Plant Dormancy (Day) | Plant Growth (Day) | Flower Bud Emergence (Day) | Fruiting (Day) |
|---|---|---|---|---|
| 2007 | 90.14 b | 267.00 a | 212.57 b | 175.71 b |
| 2008 | 91.14 a | 265.58 b | 215.14 a | 175.85 a |
| Significance | ** | ** | ** | ** |
| Biotype | Plant Dormancy (Day) | Plant Growth (Day) | Flower Bud Emergence (Day) | Fruiting (Day) |
|---|---|---|---|---|
| SCP1 | 94.00 b | 264.0 1e | 217.00 b | 178.02 d |
| SCP2 | 80.01 g | 297.02 a | 233.02 a | 181.51 a |
| SCP3 | 85.51 f | 270.51 b | 209.51 f | 177.52 e |
| SCP4 | 98.50 a | 260.01 f | 212.02 e | 178.02 c |
| SCP5 | 92.01 d | 267.02 5 | 215.01 c | 180.02 b |
| SCP6 | 91.01 e | 266.52 d | 208.02 g | 170.03 f |
| SCP7 | 93.50 c | 258.01 g | 212.52 d | 165.53 g |
| Significance | ** | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Bella, S.; Rossini, F.; Licata, M.; Virga, G.; Ruggeri, R.; Iacuzzi, N.; Leto, C.; Tuttolomondo, T. Four-Year Study on the Bio-Agronomic Response of Biotypes of Capparis spinosa L. on the Island of Linosa (Italy). Agriculture 2021, 11, 327. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11040327
La Bella S, Rossini F, Licata M, Virga G, Ruggeri R, Iacuzzi N, Leto C, Tuttolomondo T. Four-Year Study on the Bio-Agronomic Response of Biotypes of Capparis spinosa L. on the Island of Linosa (Italy). Agriculture. 2021; 11(4):327. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11040327
Chicago/Turabian StyleLa Bella, Salvatore, Francesco Rossini, Mario Licata, Giuseppe Virga, Roberto Ruggeri, Nicolò Iacuzzi, Claudio Leto, and Teresa Tuttolomondo. 2021. "Four-Year Study on the Bio-Agronomic Response of Biotypes of Capparis spinosa L. on the Island of Linosa (Italy)" Agriculture 11, no. 4: 327. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11040327