Cultivating for the Industry: Cropping Experiences with Hypericum perforatum L. in a Mediterranean Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants Management and Data Collection
2.2. Phytochemical Analyses
2.3. Statistical Treatment of Data
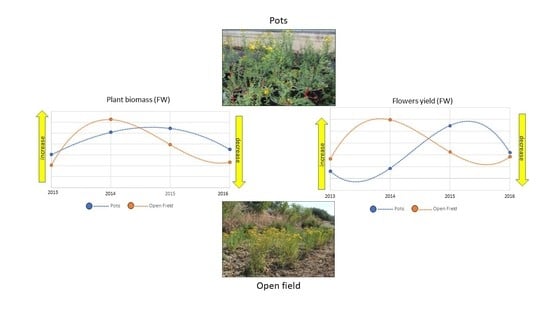
3. Results and Discussion
3.1. Plant Growth and Yield
3.2. Phytochemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Šavikin, K.; Dobrić, S.; Tadić, V.; Zdunić, G. Antiinflammatory activity of ethanol extracts of Hypericum perforatum L., H. barbatum Jacq., H. hirsutum L., H. richeri Vill. and H. androsaemum L. in rats. Phytother. Res. 2007, 21, 176–180. [Google Scholar] [CrossRef]
- Pu, X.; Liang, J.; Wang, X.; Xu, T.; Hua, L.; Shang, R.; Liu, Y.; Xing, Y. Anti-influenza A virus effect of Hypericum perforatum L. extract. Virol. Sin. 2009, 24, 19. [Google Scholar] [CrossRef]
- Cecchini, C.; Cresci, A.; Coman, M.M.; Ricciutelli, M.; Sagratini, G.; Vittori, S.; Lucarini, D.; Maggi, F. Antimicrobial activity of seven Hypericum entities from Central Italy. Planta Med. 2007, 73, 564–566. [Google Scholar] [CrossRef] [Green Version]
- Kacerovská, D.; Pizinger, K.; Majer, F.; Šmíd, F. Photodynamic therapy of nonmelanoma skin cancer with topical Hypericum perforatum extract—A pilot study. Photochem. Photobiol. 2008, 84, 779–785. [Google Scholar] [CrossRef] [PubMed]
- EMA—European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Assessment Report on Hypericum perforatum L., Herba. 2009. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-hypericum-perforatum-l-herba_en.pdf (accessed on 13 February 2021).
- EMA—European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Assessment Report on Hypericum perforatum L., Herba. 2018. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-hypericum-perforatum-l-herba-revision-1_en.pdf (accessed on 13 February 2021).
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical Application of St. John’s Wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Lazzara, S.; Carrubba, A.; Napoli, E. Variability of Hypericins and Hyperforin in Hypericum Species from the Sicilian Flora. Chem. Biodivers 2020, 17, e1900596. [Google Scholar] [CrossRef]
- ESCOP. European Scientific Cooperative on Phytotherapy. Hyperici Herba–St. John’s Wort. ESCOP Monographs, 2nd ed. Online Series. 2018. Available online: https://escop.com/downloads/hypericum-2018/ (accessed on 13 February 2021).
- Bombardelli, E.; Morazzoni, P. Hypericum perforatum . Fitoterapia 1995, 66, 43–68. [Google Scholar]
- Vitiello, B. Hypericum perforatum extracts as potential antidepressants. J. Pharm. Pharmacol. 1999, 51, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Petereit, F.; Winterhoff, H.; Nahrstedt, A. Solubilized hypericin and pseudohypericin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Med. 1998, 64, 291–294. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Bhattacharya, S.K.; Wonnemann, M.; Singer, A.; Müller, W.E. Hyperforin as a possible antidepressant component of Hypericum extracts. Life Sci. 1998, 63, 499–510. [Google Scholar] [CrossRef]
- Butterweck, V. Mechanism of Action of St John’s Wort in Depression. What is known? CNS Drugs 2003, 17, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Nahrstedt, A.; Butterweck, V. Lessons learned from herbal medicinal products: The example of St. John’s Wort. J. Nat. Prod 2010, 73, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Butterweck, V. The mechanisms of action of St. John’s wort: An update. Wien Med. Wochenschr. 2015, 165, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.L. Essential oil and volatile components of the genus Hypericum (Hypericaceae). Nat. Prod. Commun. 2010, 5, 1493–1506. [Google Scholar] [CrossRef] [Green Version]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Cyrak, C. Seed germination protocols for ex situ conservation of some Hypericum species from Turkey. Am. J. Plant Physiol. 2007, 2, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Lazzara, S.; Landini, E.; Saia, S.; Fascella, G.; Carrubba, A. Preliminary studies about sexual and vegetative propagation of Hypericum perforatum in a Mediterranean environment. In Proceedings of the X Convegno Nazionale sulla Biodiversità, Roma, Italy, 3–5 September 2014; pp. 206–211. [Google Scholar]
- Fascella, G.; Airò, M.; Mammano, M.M.; Giardina, G.; Carrubba, A.; Lazzara, S. Rooting and acclimatization of micropropagated Hypericum perforatum L. native to Sicily. Acta Hort. 2017, 1155, 543–548. [Google Scholar] [CrossRef]
- NRCS. Keys to Soil Taxonomy, 9th ed.; USDA NRCS, Soil Survey Staff: Washington, DC, USA, 2003; 332p.
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø. PAST 4.03. 2020. Available online: https://www.nhm.uio.no/english/research/infrastructure/past/ (accessed on 13 February 2020).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Kizil, S.; Inan, M.; Kirici, S. Determination of the best herbage yield and hypericin content of St. John’s wort (Hypericum perforatum L.) under semi-arid climatic conditions. Turk. J. Field Crops 2013, 18, 95–100. [Google Scholar]
- Pluhár, Z.S.; Bernáth, J.; Neumayer, É. Morphological, production biological and chemical diversity of St. John’s Wort (Hypericum perforatum L.). Acta Hort. 2002, 576, 33–40. [Google Scholar] [CrossRef]
- Giovino, A.; Carrubba, A.; Lazzara, S.; Napoli, E.; Domina, G. An integrated approach to the study of Hypericum occurring in Sicily. Turk. J. Bot. 2020, 44, 309–321. [Google Scholar] [CrossRef]
- Maisenbacher, P.; Kovar, K.A. Analysis and stability of Hyperici oleum. Planta Med. 1992, 58, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, P.; Altschmied, L.; Ravindran, B.M.; Rutten, T.; D’Auria, J.C. The biochemical and genetic basis for the biosynthesis of bioactive compounds in Hypericum perforatum L., one of the largest medicinal crops in Europe. Genes 2020, 11, 1210. [Google Scholar] [CrossRef]
- Michler, H.; Laakmann, G.; Wagner, H. Development of an LC-MS method for simultaneous quantitation of Amentoflavone and Biapigenin, the minor and major biflavones from Hypericum perforatum L., in human plasma and its application to real blood. Phytochem. Anal. 2011, 22, 42–50. [Google Scholar] [CrossRef]
- Robson, N.K.B. Studies in the genus Hypericum L. (Guttiferae) 4 (2). Section 9. Hypericum sensu lato (part 2): Subsection 1. Hypericum series 1. Hypericum. Bull. Br. Mus. Nat. Hist. Bot. 2002, 32, 61–123. [Google Scholar] [CrossRef]
- Büter, B.; Orlacchio, C.; Soldati, A.; Berger, K. Significance of genetic and environmental aspects in the field cultivation of Hypericum perforatum. Planta Med. 1998, 64, 431–437. [Google Scholar] [CrossRef] [PubMed]







| Source of Variability | DF | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| Management (M) | 1 | <1 n.s. | 33.67 *** | 2.14 n.s. | 3.52 n.s. |
| Biotype (B) | 2 | 111.94 *** | 162.66 *** | 1.71 n.s. | <1 n.s. |
| M × B | 2 | <1 n.s. | <1 n.s. | <1 n.s. | 1.03 n.s. |
| Error | 24 | ||||
| Total | 29 |
| Source of Variability | DF | Number of Stems (no.) | Weight of Stems (g) | |||
|---|---|---|---|---|---|---|
| with Flowers | Vegetative | Total | FW | DW | ||
| Year (Y) | 3 | <1 n.s. | 2.65 n.s. | <1 n.s. | 7.69 n.s. | 4.15 n.s. |
| Management (M) | 1 | 1.10 | <1 n.s. | <1 n.s. | 16.36 * | 5.00 n.s. |
| Biotype (B) | 2 | <1 n.s. | 15.73 ** | <1 n.s. | 2.37 n.s. | 5.94 * |
| Y × M | 3 | 3.27 n.s. | 1.7 n.s. | 4.87 * | <1 n.s. | <1 n.s. |
| Y × B | 6 | 2.52 n.s. | 1.53 n.s. | 4.1 n.s. | 1.31 n.s. | 1.38 n.s. |
| M × B | 2 | 10.16 * | 1.4 n.s. | 8.22 * | 3.34 n.s. | 4.81 n.s. |
| Y × M × B | 6 | 3.28 *** | 1.29 n.s. | 1.67 n.s. | 7.27 *** | 5.40 *** |
| Error | 96 | |||||
| Total | 119 | |||||
| Source of Variability | DF | 2013 | 2014 | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|---|---|---|
| FW | DW | FW | DW | FW | DW | FW | DW | ||
| Management (M) | 1 | 8.18 ** | 14.77 *** | 1.96 n.s. | 4.66 * | 19.91 *** | 5.94 * | 11.12 ** | 6.58 * |
| Biotype (B) | 2 | 52.00 *** | 59.67 *** | 4.84 * | 10.65 *** | 38.04 *** | 28.79 *** | 2.01 n.s. | 2.31 n.s. |
| M × B | 2 | 9.03 *** | 12.97 *** | 13.11 *** | 8.05 ** | 13.25 *** | 8.73 *** | <1 n.s. | <1 n.s. |
| Error | 24 | ||||||||
| Total | 29 | ||||||||
| Source of Variability | DF | 2013 | 2014 | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|---|---|---|
| FW | DW | FW | DW | FW | DW | FW | DW | ||
| Management (M) | 1 | 24.17 *** | 6.42 * | 31.26 *** | 29.50 *** | 104.10 *** | 35.69 *** | 8.56 ** | 1.32 n.s. |
| Biotype (B) | 2 | 34.54 *** | 20.89 *** | 13.97 *** | 13.60 *** | 38.74 *** | 48.31 *** | <1 n.s. | 1.59 n.s. |
| M × B | 2 | 18.34 *** | 12.93 *** | 11.56 *** | 10.66 *** | 25.34 *** | 22.30 *** | 5.81 ** | 2.96 n.s. |
| Error | 24 | ||||||||
| Total | 29 | ||||||||
| Loadings | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 |
|---|---|---|---|---|---|---|---|
| Cat | 0.54750 | 0.59633 | −0.01455 | 0.27531 | −0.03179 | 0.51733 | 1.97 × 10−14 |
| Phlor | 0.98032 | −0.19132 | −0.04742 | −0.00513 | −0.00964 | −0.00125 | 3.02 × 10−16 |
| Napht | 0.69665 | −0.40432 | 0.58406 | −0.09964 | −0.01061 | −0.00619 | 1.7 × 10−15 |
| Cinn | −0.48407 | 0.56480 | −0.33371 | −0.16055 | 0.55611 | −0.01671 | 4.41 × 10−15 |
| Flav | −0.11142 | 0.98737 | −0.07198 | −0.06860 | −0.05277 | −0.00416 | 9.72 × 10−16 |
| Dim | 0.66202 | 0.63131 | 0.11850 | 0.38590 | −0.00336 | −0.01434 | 2.23 × 10−15 |
| Phen | 0.98775 | 0.15402 | 0.02353 | 0.00109 | 0.00800 | 0.00106 | −2.6 × 10−16 |
| Eigenvalues | 1029.890 | 79.784 | 6.816 | 1.657 | 0.860 | 0.034 | 2.43 × 10−18 |
| % variance | 92.03 | 7.13 | 0.61 | 0.15 | 0.08 | 0.00 | 2.17 × 10−19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzara, S.; Carrubba, A.; Napoli, E. Cultivating for the Industry: Cropping Experiences with Hypericum perforatum L. in a Mediterranean Environment. Agriculture 2021, 11, 446. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11050446
Lazzara S, Carrubba A, Napoli E. Cultivating for the Industry: Cropping Experiences with Hypericum perforatum L. in a Mediterranean Environment. Agriculture. 2021; 11(5):446. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11050446
Chicago/Turabian StyleLazzara, Silvia, Alessandra Carrubba, and Edoardo Napoli. 2021. "Cultivating for the Industry: Cropping Experiences with Hypericum perforatum L. in a Mediterranean Environment" Agriculture 11, no. 5: 446. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11050446