Progress in Almond Quality and Sensory Assessment: An Overview
Abstract
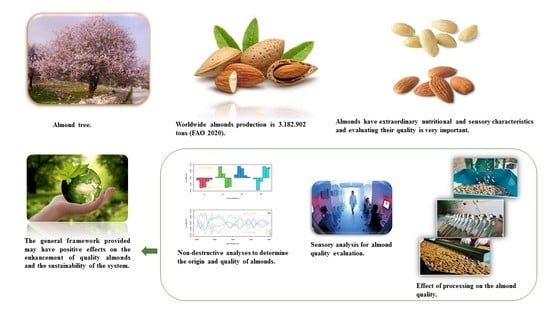
:1. Introduction
2. Almond Fruit Characteristics
3. Chemical Composition and Nutritional Value
4. Antioxidant Compounds
5. Processing Influence on Almond Quality
6. Sensory Analysis
7. Quality of Almonds Valued by Non-Destructive Analysis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- R. Socias i Company; Ansón, J.M.; Espiau, M.T.; Gradziel, T.M. Taxonomy, botany and physiology. In Almonds: Botany, Production and Uses; R. Socias i Company, Gradziel, T.M., Eds.; CAB International: Boston, MA, USA, 2017; pp. 1–42. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Hojjati, M.; Lipan, L.; Carbonell-Barrachina, A. Effect of Roasting on Physicochemical Properties of Wild Almonds (Amygdalus scoparia). J. Am. Oil Chem. Soc. 2016, 93, 1211–1220. [Google Scholar] [CrossRef]
- Zeinalabedini, M.; Khayam-Nekoui, M.; Grigorian, V.; Gradziel, T.M.; MartínezGómez, P. The origin and dissemi-nation of the cultivated almond as determined by nuclear and chloroplast SSR marker analysis. Sci. Hortic. 2010, 125, 593–601. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Cigliano, R.A.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Dicenta, F.; López-Marqués, R.L.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M.; Curtis, R.; R. Socias i Company. Production and Growing Regions; CABI Press: Boston, MA, USA, 2017; pp. 70–86. [Google Scholar] [CrossRef]
- Gradziel, T.M. Redomesticating Almond to Meet Emerging Food Safety Needs. Front. Plant Sci. 2020, 11, 778. [Google Scholar] [CrossRef]
- Ladizinsky, G. On the origin of almond. Genet. Resour. Crop Evol. 1999, 46, 143–147. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe, and the Nile Valley; Oxford University Press: Oxford, UK, 2000; Volume 186, ISBN 0-19-850356-3. [Google Scholar]
- Delplancke, M.; Alvarez, N.; Benoit, L.; Espi´Ndola, A.; Joly, H.I.; Neuenschwander, S.; Arrigo, N. Evolutionary history of almond tree domestication in the Mediterranean basin. Mol. Ecol. 2013, 22, 1092–1104. [Google Scholar] [CrossRef]
- Gray, J. Nuts and Seeds. In Encyclopedia of Human Nutrition; Academic Press: London, UK, 2015; pp. 381–388. [Google Scholar]
- Mirrahimi, A.; Srichaiku, K.; Esfahani, A.; Banach, M.S.; Sievenpiper, J.L.; Kendall, G.W.C.; Jenkins, D.J.A. Chapter 8 Almond (Prunus dulcis) Seeds and Oxidative Stress. In Nuts & Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 161–166. [Google Scholar]
- FAO. Food and Agriculture Organization of United Nations. 2020. Available online: http://faostat.fao.org/en/#data/QC (accessed on 15 June 2020).
- International Nut & Dried Fruit Council. Nuts & Dried Fruits Statistical Yearbook; Carrer de la Fruita Seca; 4 Polígon Tecnoparc: Reus, Spain, 2021. [Google Scholar]
- Grundy, M.; Lapsley, K.; Ellis, P.R. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int. J. Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef] [Green Version]
- Kalita, S.; Khandelwal, S.; Madan, J.; Pandya, H.; Sesikeran, B.; Krishnaswamy, K. Almonds and Cardiovascular Health: A Review. Nutrients 2018, 10, 468. [Google Scholar] [CrossRef] [Green Version]
- Franklin, L.M.; Mitchell, A.E. Review of the Sensory and Chemical Characteristics of Almond (Prunus dulcis) Flavor. J. Agric. Food Chem. 2019, 67, 2743–2753. [Google Scholar] [CrossRef] [Green Version]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Review about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Verma, M.K. Scientific Almond Cultivation for Higher Returns Central Institute of Temperate Horti-Culture; J&K: Srinagar, India, 2009. [Google Scholar]
- Egea, G.; Nortes, P.; Domingo, R.; Baille, A.; Pastor, A.P.; González-Real, M.M. Almond agronomic response to long-term deficit irrigation applied since orchard establishment. Irrig. Sci. 2012, 31, 445–454. [Google Scholar] [CrossRef]
- Doll, D. Impacts of drought on almond production. West. Fruit Grow. 2014, 134, 7. [Google Scholar]
- Lipan, L.; Sánchez Rodríguez, L.; Collado González, J.; Sendra, E.; Burló, F.; Hernández, F.; Vodnar, D.C.; Carbonell Bar-rachina, A.A. Sustainability of the legal endowments of water in almond trees and a new generation of high quality hydrosustainable almonds—A review. Bull. UASVM Food Sci. Technol. 2018, 75, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Lipan, L.; Cano-Lamadrid, M.; Hernández, F.; Sendra, E.; Corell, M.; Vázquez-Araújo, L.; Moriana, A.; Carbonell-Barrachina, A.A. Long-Term Correlation betweenWater Deficit and Quality Markers in HydroSOStainable Almonds. Agronomy 2020, 10, 1470. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Sánchez-Pérez, R.; Dicenta, F.; Howad, W.; Arús, P.; Gradziel, T. Almond. In Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 229–242. [Google Scholar]
- Halasz, J.; Kodad, O.; Galiba, G.M.; Skola, I.; Ercisli, S.; Ledbetter, C.A.; Hegedűs, A. Genetic variability is preserved among strongly differentiated and geographically diverse almond germplasm: An assessment by simple sequence repeat markers. Tree Genet. Genomes 2019, 15, 12. [Google Scholar] [CrossRef] [Green Version]
- UPOV. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability; Almond (Prunus dulcis (Mill.) TG/56/4 Corr. Rev; International Union for the Protection of New Varieties of Plants: Geneve, Switzerland, 2019.
- Cobos, F.P.D.L.; Martínez-García, P.J.; Romero, A.; Miarnau, X.; Eduardo, I.; Howad, W.; Mnejja, M.; Dicenta, F.; Company, R.S.; Rubio-Cabetas, M.J.; et al. Pedigree analysis of 220 almond genotypes reveals two world mainstream breeding lines based on only three different cultivars. Hortic. Res. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- De Giorgio, D.; Leo, L.; Zacheo, G.; Lamascese, N. Evaluation of 52 almond (Prunus amygdalus Batsch) cultivars from the Apulia region in Southern Italy. J. Hortic. Sci. Biotechnol. 2007, 82, 541–546. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Pantelidis, G.; Bacchetta, L.; de Giorgio, D.; Duval, H.; Metzidakis, I.; Spera, D. Protein and mineral nutrient contents in kernels from 72 sweet almond cultivars and accessions grown in France, Greece and Italy. Int. J. Food Sci. Nutr. 2012, 64, 202–209. [Google Scholar] [CrossRef]
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the chemical and nutritional characteristics of almonds (Prunus dulcis (Mill). D.A.Webb) as influenced by harvest time and cultivar. J. Sci. Food Agric. 2018, 98, 5647–5655. [Google Scholar] [CrossRef] [Green Version]
- Beltrán Sanahuja, A.; Maestre Pérez, S.E.; Grané Teruel, N.; Valdés García, A.; Prats Moya, M.S. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods 2021, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Gouta, H.; Ksia, E.; Laaribi, I.; Molino, F.; Estopañan, G.; Juan, T.; Kodad, O.; Martínez-Gómez, P.; Martínez-García, P. Evaluation of the chemical and nutritional properties of tunisian almond cultivars. Ital. J. Food Sci. 2020, 32, 562–582. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Yousefi, R.; Tran, L.-S.P. Physical and biochemical properties of 10 wild almond (Amygdalus scoparia) accessions naturally grown in Iran. Food Biosci. 2020, 37, 100721. [Google Scholar] [CrossRef]
- Yada, S.; Huang, G.; Lapsley, K. Natural variability in the nutrient composition of California-grown almonds. J. Food Compos. Anal. 2013, 30, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Simsek, M.; Gulsoy, E.; Yavic, A.; Arikan, B.; Yildirim, Y.; Olmez, N.; Erdogmus, B.; Boguc, F. Fatty acid, mineral and proximate compositions of various genotypes and commercial cultivars of sweet almond from the same ecological conditions. Appl. Ecol. Environ. Res. 2018, 16, 2957–2971. [Google Scholar] [CrossRef]
- Kodad, O.; Socias i Company, R.; Estopañán, G.; Juan, T.; Molino, F.; Mamouni, A.; Messaoudi, Z.; Lahlo, M. Plasticity and stability of major fatty acids in almond cultivars under Mediterranean climate. J. Hortic. Sci. Bio-Tecnol. 2010, 85, 381–386. [Google Scholar] [CrossRef]
- USDA. National Nutrient Database for Standard Reference. 2019. Available online: https://www.nal.usda.gov/ (accessed on 2 April 2022).
- I Forcada, C.F.; Kodad, O.; Juan, T.; Estopañán, G.; Rafel Socias i Company. Genetic variability and pollen effect on the transmission of the chemical components of the almond kernel. Span. J. Agric. Res. 2011, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Kodad, O.; Estopañán, G.; Juan, T.; R. Socias i Company. Protein Content and Oil Composition of Almond from Moroccan Seedlings: Genetic Diversity, Oil Quality and Geographical Origin. J. Am. Oil Chem. Soc. 2012, 90, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Yıldırım, A.N.; Yildirim, F.; Şan, B.; Polat, M.; Sesli, Y. Variability of phenolic composition and tocopherol content of some commercial Almond cultivars. J. Appl. Bot. Food Qual. 2016, 89, 163–170. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R. Properties of biological activity of ten wild almond (Prunus amygdalus L.) species. Turk. J. Biol. 2012, 36, 201–209. [Google Scholar]
- Kodad, O.; Alonso, J.M.; Espiau, M.T.; Estopañán, G.; Juan, T.; Rafel Socias i Company. Chemometric Characterization of Almond Germplasm: Compositional Aspects Involved in Quality and Breeding. J. Am. Soc. Hortic. Sci. 2011, 136, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Izaddost, M.; Imani, A.; Piri, S.; Bagiri, A.M. Oil content, major fatty acids composition, γ-tocopherol, β-tocopherol and nut characteris¬tics of almond at time of harvest. J. Basic Appl. Sci. Res. 2013, 3, 201–205. [Google Scholar]
- Zhu, Y.; Wilkinson, K.; Wirthensohn, M. Lipophilic antioxidant content of almonds (Prunus dulcis): A regional and varietal study. J. Food Compos. Anal. 2015, 39, 120–127. [Google Scholar] [CrossRef]
- Kodad, O.; Socias i Company, R.; Alonso, J.M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallico, B.; Ballistreri, G.; Arena, E.; Tokusoglu, O. Nut Bioactives: Phytochemicals and Lipid-Based Components of Almonds, Hazelnuts, Peanuts, Pistachios, and Walnuts. In Fruit and Cereal Bioactives: Sources, Chemistry, and Applications; Taylor & Francis Group: Oxford, UK, 2011. [Google Scholar]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, A.G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol content and antioxidant activity of Cali-fornia almonds depend on cultivar and harvest year. Food Chem. 2011, 123, 1040–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Zhong, Y.; Wijeratne, S.S.K.; Ho, C.-H. Almond and almond products: Nutraceutical components and health effects. In Tree Nuts: Composition, Phytochemicals, and Health Effects; Alasalvar, C., Shahidi, F., Eds.; Taylor & Francis Group, LLC: Oxford, UK, 2009. [Google Scholar]
- Bolling, B.W. Almond polyphenols: Methods of analysis, contribution to food quality, and health promotion. Com-Prehensive Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, A.; Khaliq, M.; Saeed, A.; Azeem, M.W.; Ghania, J.B. Almond (Purunus amygdalus L.): A review on health benefits, nutritional value and therapeutic applications. Int. J. Chem. Biochem. Sci. 2015, 8, 103–106. [Google Scholar]
- Lee-Bravatti, M.A.; Wang, J.; Avendano, E.E.; King, L.; Johnson, E.J.; Raman, G. Almond Consumption and Risk Factors for Cardiovascular Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2019, 10, 1076–1088. [Google Scholar] [CrossRef]
- Hollingworth, S.; Dalton, M.; Blundell, J.E.; Finlayson, G. Evaluation of the Influence of Raw Almonds on Appetite Control: Satiation, Satiety, Hedonics and Consumer Perceptions. Nutrients 2019, 11, 2030. [Google Scholar] [CrossRef] [Green Version]
- Ben-Nun, L. Health Properties of Almonds; B. N. Publication House: Beer-Sheva, Israel, 2020. [Google Scholar]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.-M.; Mocan, A.; Vlase, L.; Popa, D.-S.; Ferreira, I.C.F.R. Benefits of tree nut consumption on aging and age-related diseases: Mechanisms of actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- Li, Z.; Bhagavathula, A.S.; Batavia, M.; Clark, C.; Abdulazeem, H.M.; Rahmani, J.; Yin, F. The effect of almonds consumption on blood pressure: A systematic review and dose-response meta-analysis of randomized control trials. J. King Saud Univ.-Sci. 2020, 32, 1757–1763. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodad, O.; Socias i Company, R.; Prats, M.S.; Ortiz, M.C.L. Variability in tocopherol concentrations in almond oil and its use as a selection criterion in almond breeding. J. Hortic. Sci. Biotechnol. 2006, 81, 501–507. [Google Scholar] [CrossRef]
- Zhu, Y. Almond (Prunus dulcis (Mill.) D.A. Webb) Fatty Acids and Tocopherols under Different Conditions. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2014. [Google Scholar]
- Maestri, D.; Martinez, M.L.; Bodoira, R.; Rossi, Y.; Oviedo, A.; Pierantozzi, P.; Torres, M. Variability in almond oil chemical traits from traditional cultivars and native genetic resources from Argentina. Food Chem. 2015, 170, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gordillo, S.; Lipan, L.; Zuazo, V.H.D.; Sendra, E.; Hernández, F.; Hernández-Zazueta, M.S.; Carbonell-Barrachina, A.; García-Tejero, I.F. Deficit Irrigation as a Suitable Strategy to Enhance the Nutritional Composition of HydroSOS Almonds. Water 2020, 12, 3336. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Verdú, A.; Navarro, P.; Martínez-Sánchez, F.; Carbonell-Barrachina, A. Changes in volatile compounds and sensory quality during toasting of Spanish almonds. Int. J. Food Sci. Technol. 2009, 44, 2225–2233. [Google Scholar] [CrossRef]
- Bolling, B.; Blumberg, J.B.; Chen, C.-Y.O. The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chem. 2010, 123, 1040–1047. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS character-ization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Perez, R.; Jorgensen, K.; Olsen, C.E.; Dicenta, F.; Moller, B.L. Bitterness in almonds. Plant Physiol. 2008, 146, 1040–1052. [Google Scholar] [CrossRef] [Green Version]
- Nizamlioglu, N.M.; Nas, S. Kinetic of Color Changes in Almond (Akbadem Variety) During Roasting and Storage. Int. J. Food Prop. 2016, 19, 2363–2376. [Google Scholar] [CrossRef] [Green Version]
- Kaftan, A. Kinetics of Color Degradation in Thermal Processed Almond. In Proceedings of the International Conference on Environment, Energy, and Biotechnology, IPCBEE, Kuala Lumpur, Malaysia, 5–6 May 2012; IACSIT Press: Singapore, 2012; Volume 33. [Google Scholar]
- Makinde, F.M.; Oladunni, S.S. Effects of processing treatments on nutritional quality of raw almond (Terminalia catappa Linn.) kernels. Adv. Appl. Sci. Res. 2016, 7, 1–7. [Google Scholar]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of different processing treatments on almond (Prunus dulcis) bioactive compounds, antioxidant activities, fatty acids, and sensorial characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the oxidative stability and volatiles in blanched, roasted and fried almonds under normal and accelerated storage conditions by DSC, thermogravimetric analysis and ATR-FTIR. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213. [Google Scholar] [CrossRef]
- Caltagirone, C.; Peano, C.; Sottile, F. Post-harvest Industrial Processes of Almond (Prunus dulcis L. Mill) in Sicily Influence the Nutraceutical Properties of By-Products at Harvest and During Storage. Front. Nutr. 2021, 8, 659378. [Google Scholar] [CrossRef]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Progress in evaluating chestnuts quality: A review of recent develop-ments. Trends Food Sci. Technol. 2021, 113, 245–254. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, S.C.; Hu, C.C.; Shyu, Y.S.; Hsu, C.Y.; Yang, D.J. Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef]
- Bartolomé, B.; Garrido, I.; Monagas, M.; Gómez-Cordovés, C. Extraction of antioxidants from almond-processing byproducts. Grasas y Aceites 2007, 58, 130–135. [Google Scholar] [CrossRef]
- Sruthi, N.; Premjit, Y.; Pandiselvam, R.; Kothakota, A.; Ramesh, S. An overview of conventional and emerging techniques of roasting: Effect on food bioactive signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.; Salvador, A.; Fiszman, S. On the assessment of fracture in brittle foods: The case of roasted almonds. Food Res. Int. 2008, 41, 544–551. [Google Scholar] [CrossRef]
- Civille, G.; Lapsley, K.; Huang, G.; Yada, S.; Seltsam, J. Development of an almond lexicon to assess the sensory properties of almond varieties. J. Sens. Stud. 2010, 25, 146–162. [Google Scholar] [CrossRef]
- Vickers, Z.; Peck, A.; Labuza, T.; Huang, G. Impact of Almond Form and Moisture Content on Texture Attributes and Acceptability. J. Food Sci. 2014, 79, S1399–S1406. [Google Scholar] [CrossRef] [PubMed]
- Contador, L.; Robles, B.; Shinya, P.; Medel, M.; Pinto, C.; Reginato, G.; Infante, R. Characterization of texture attributes of raw almond using a trained sensory panel. Fruits 2015, 70, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Padehban, L.; Ansari, S.; Koshani, R. Effect of packaging method, temperature and storage period on physicochemical and sensory properties of wild almond kernel. J. Food Sci. Technol. 2018, 55, 3408–3416. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.; Corell, M.; Sendra, E.; Hernández, F.; Stan, L.; Vodnar, D.C.; Vázquez-Araújo, L.; Carbonell-Barrachina, A. Sensory Profile and Acceptability of HydroSOStainable Almonds. Foods 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- King, E.S.; Chapman, D.M.; Luo, K.; Ferris, S.; Huang, G.; Mitchell, A.E. Defining the Sensory Profiles of Raw Almond (Prunus dulcis) Varieties and the Contribution of Key Chemical Compounds and Physical Properties. J. Agric. Food Chem. 2019, 67, 3229–3241. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Newman, L.; Gangadoo, S.; Pathirannahalage, P.R.; Cheeseman, S.; Cozzolino, D. Sensomics—From conventional to functional NIR spectroscopy—Shining light over the aroma and taste of foods. Trends Food Sci. Technol. 2019, 91, 274–281. [Google Scholar] [CrossRef]
- Firmani, P.; La Piscopia, G.; Bucci, R.; Marini, F.; Biancolillo, A. Authentication of P.G.I. Gragnano pasta by near infrared (NIR) spectroscopy and chemometrics. Microchem. J. 2019, 152, 104339. [Google Scholar] [CrossRef]
- Corona, P.; Frangipane, M.T.; Moscetti, R.; Feudo, G.L.; Castellotti, T.; Massantini, R. Chestnut Cultivar Identification through the Data Fusion of Sensory Quality and FT-NIR Spectral Data. Foods 2021, 10, 2575. [Google Scholar] [CrossRef]
- Arndt, M.; Rurik, M.; Drees, A.; Bigdowski, K.; Kohlbacher, O.; Fischer, M. Comparison of different sample prepa-ration techniques for NIR screening and their influence on the geographical origin determination of almonds (Prunus dulcis MILL.). Food Control 2020, 115, 107302. [Google Scholar] [CrossRef]
- Arndt, M.; Rurik, M.; Drees, A.; Ahlers, C.; Feldmann, S.; Kohlbacher, O.; Fischer, M. Food authentication: Deter-mination of the geographical origin of almonds (Prunus dulcis MILL.) via near-infrared spectroscopy. Microchem. J. 2021, 160, 105702. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Perez, M.; Lohumi, S.; Lee, H.; Kim, G.; Wakholi, C.; Joshi, R.; Cho, B.-K. Online Application of a Hyperspectral Imaging System for the Sorting of Adulterated Almonds. Appl. Sci. 2020, 10, 6569. [Google Scholar] [CrossRef]
- Torres, I.; Pérez-Marín, D.; Vega-Castellote, M.; Sánchez, M.-T. Mapping of fatty acids composition in shelled almonds analysed in bulk using a Hyperspectral Imaging system. LWT 2020, 138, 110678. [Google Scholar] [CrossRef]
- Vega-Castellote, M.; Pérez-Marín, D.; Torres, I.; Moreno-Rojas, J.M.; Sànchez, M.T. Exploring the potential of NIRS technology for the insitu prediction of amygdalin content and classification by bitterness of in-shell and shelled intact almonds. J. Food Eng. 2021, 294, 110406. [Google Scholar] [CrossRef]
- Vega-Castellote, M.; Sánchez, M.-T.; Torres, I.; Pérez-Marín, D. An innovative non-targeted control system based on NIR spectral information for detecting non-compliant batches of sweet almonds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 250, 119407. [Google Scholar] [CrossRef]

| Almond Cultivars | Total Lipids Content (mg/g) | α-Tocopherol (μg/g) | Oleic/ Linoleic Ratio | Unsaturated Fatty Acids % | Carbohydrate Content (g/kg Fresh Weight) | Antioxidant Activity (µmol Trolox Equivalents Per Gram) | K (mg/ 100 g dm) | P (mg/ 100 g dm) | Mg (mg/ 100 g dm) | Ca (mg/ 100 g dm) | Total Polyphenols (mg/kg) | Protein (g/100 g) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 almond cultivars from the Apulia region of Italy | 450–633 | 218–777 | De Giorgio et al., 2007 [28] | ||||||||||
| 72 almond genotypes from France, Greece and Italy | 488–1235 | 310–748 | 159–321 | 206–663 | 10–29 | Drogoudi et al., 2012 [29] | |||||||
| 7 almond cultivars from California | 219–310 | 664–773 | 462–526 | 256–278 | 234–330 | 20.2–22.5 | Yada et al., 2013 [34] | ||||||
| 10 genotypes and 2 commercial cultivars (Ferragnes and Ferraduel) from Turkey | 90.27–92.09 | 679.53–986.63 | 584.37–697.31 | 225.27–381.93 | 189.63–332.19 | 20.41–25.82 | Simsek et al., 2018 [35] | ||||||
| 10 almond cultivars from Australia, California, Italy and Spain | 423.9–561.7 | 157.1–266.3 | 12.69–60.99 | 391.98–11030.53 | 14.12–22.08 | Summo et al., 2018 [30] | |||||||
| 16 almond cultivars including 12 from Tunisia, 2 from Italy, 1 from Spain and 1 from France | 477.5–609.5 | 2.76–5.67 | 88.38–91.65 | 14.49–27.15 | Gouta et al., 2020 [32] | ||||||||
| 10 wild almond Iranian accessions | 570–890 | 430–700 | 220–400 | 210–370 | Zahedi et al., 2020 [33] |
| Name | Amount | Unit |
|---|---|---|
| Water | 4.41 | g |
| Energy | 579 | kcal |
| Protein | 21.2 | g |
| Total lipids (fat) | 49.9 | g |
| Ash | 2.97 | g |
| Total sugars | 4.35 | g |
| Calcium, Ca | 269 | g |
| Iron, Fe | 3.71 | mg |
| Magnesium, Mg | 270 | mg |
| Phosphorus, P | 481 | mg |
| Potassium, K | 733 | mg |
| Zinc, Zn | 3.12 | mg |
| Selenium, Se | 4.1 | mg |
| Riboflavin | 1.14 | mg |
| Niacin | 3.62 | mg |
| Pantothenic acid | 0.471 | mg |
| Vitamin B-6 | 0.137 | mg |
| Folate, total | 44 | mg |
| Vitamin E (alpha-tocopherol) | 25.6 | mg |
| Fatty acids, total saturated | 3.8 | g |
| Fatty acids, total monounsaturated | 31.6 | g |
| Fatty acids, total polyunsaturated | 12.3 | g |
| Cholesterol | 0 | mg |
| Beta-sitosterol | 130 | mg |
| Tryptophan | 0.211 | g |
| Threonine | 0.601 | g |
| Isoleucine | 0.751 | g |
| Leucine | 1.47 | g |
| Lysine | 0.568 | g |
| Methionine | 0.157 | g |
| Cystine | 0.215 | g |
| Phenylalanine | 1.13 | g |
| Tyrosine | 0.45 | g |
| Valine | 0.855 | g |
| Arginine | 2.46 | g |
| Histidine | 0.539 | g |
| Alanine | 0.999 | g |
| Aspartic acid | 2.64 | g |
| Glutamic acid | 6.21 | g |
| Glycine | 1.43 | g |
| Proline | 0.969 | g |
| Serine | 0.912 | g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massantini, R.; Frangipane, M.T. Progress in Almond Quality and Sensory Assessment: An Overview. Agriculture 2022, 12, 710. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture12050710
Massantini R, Frangipane MT. Progress in Almond Quality and Sensory Assessment: An Overview. Agriculture. 2022; 12(5):710. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture12050710
Chicago/Turabian StyleMassantini, Riccardo, and Maria Teresa Frangipane. 2022. "Progress in Almond Quality and Sensory Assessment: An Overview" Agriculture 12, no. 5: 710. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture12050710