Macro and Micronutrient Storage in Plants and Their Remobilization When Facing Scarcity: The Case of Drought
Abstract
:1. Introduction
2. General Patterns of Nutrient Mobilization from Leaves
3. Short-Term Mobilization of Stored Compounds When Facing Nutrient Deficiencies or High Nutrient Needs
4. Monocarpic Senescence and Remobilization of Nutrients
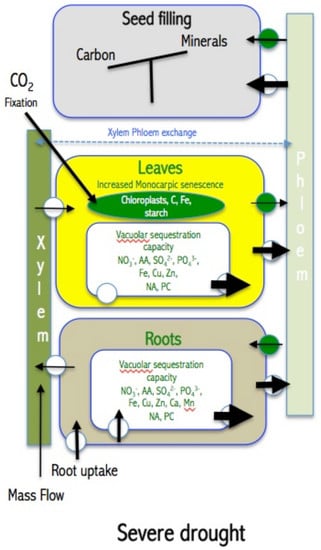
5. How Drought Affects Leaf Accumulation of Nutrients, Leaf Senescence, Mobilization of Nutrients and Seed Filling
6. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gojon, A.; Nacry, P.; Davidian, J.C. Root uptake regulation: A central process for NPS homeostasis in plants. Curr. Opin. Plant Biol. 2009, 12, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Jiménez, M.; Galván, A.; Fernández, E.; Llamas, A. Homeostasis of the micronutrients Ni, Mo and Cl with specific biochemical functions. Curr. Opin. Plant Biol. 2009, 12, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zhou, J.; Chen, J.; Zhu, L.; Zhao, Y.; Huang, Y. The genetic architecture of zinc and iron content in maize grains as revealed by QTL mapping and meta-analysis. Breed. Sci. 2013, 63, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Batten, G.D.; Crowler, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Fan, M.S.; Zhao, F.J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Luthi, D.; Litschi, M.; Schar, C. Land-atmosphere coupling and climate change in Europe. Nature 2006, 443, 205–209. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Gruber, B.D.; von Wirén, N. It is time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2013, 65, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.D.; Hawkins, H.J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Avice, J.-C.; Etienne, P. Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica Napus L.). J. Exp. Bot. 2014, 65, 3813–3824. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H. Senescence, ageing and death of the whole plant. New Phytol. 2013, 197, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Pottier, M.; Masclaux Daubresse, C.; Yoshimoto, K.; Thomine, S. Autophagy as a possible mechanism for micronutrient remobilization from leaves to seeds. Front. Plant Sci. 2014, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.M.; Schmidt, R.; Wagstaff, C.; Jing, H.C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Pearson, J.N.; Marentes, E. The physiology of micronutrient homeostasis in field crops. Field Crops Res. 1999, 60, 41–56. [Google Scholar] [CrossRef]
- Waters, B.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Ricachenevsky, F.K.; Williams, L.E.; Vasconcelos, M.W.; Menguer, P.K. From soil to seed: Micronutrient movement into and within the plant. Front. Plant Sci. 2014, 5, 438. [Google Scholar] [CrossRef] [PubMed]
- Salon, C.; Avice, J.C.; Colombi, S.; Dieuaide-Noubhani, M.; Gallardo, K.; Jeudy, C.; Ourry, A.; Prudent, M.; Voisin, A.S.; Rolin, D. Fluxomics links cellular functional analyses to whole-plant phenotyping. J. Exp. Bot. 2017, 68, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Haslett, B. Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Impa, S.M.; Johnson-Beebout, S.E. Mitigating zinc deficiency and achieving high grain Zn in rice through integration of soil chemistry and plant physiology research. Plant Soil 2012, 361, 3–41. [Google Scholar] [CrossRef]
- Riesen, O.; Feller, U. Redistribution of nickel, cobalt, manganese, zinc, and cadmium via the phloem in young and maturing wheat. J. Plant Nutr. 2005, 28, 421–430. [Google Scholar] [CrossRef]
- Drossopoulos, J.B.; Bouranis, D.L.; Bairaktari, B.D. Patterns of mineral nutrient fluctuations in soybean leaves in relation to their position. J. Plant Nutr. 1995, 117, 1017–1035. [Google Scholar] [CrossRef]
- Himelblau, E.; Amasino, R.M. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J. Plant Physiol. 2001, 158, 1317–1323. [Google Scholar] [CrossRef]
- Sperotto, R.A.; Vasconcelos, M.W.; Grusak, M.A.; Fett, J.P. Effects of different Fe supplies on mineral partitioning and remobilization during the reproductive development of rice (Oryza Sativa L.). Rice 2012, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A. Leaf mineral nutrient remobilization during Leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.P.; Grusak, M.A. Whole shoot mineral partitioning and accumulation in pea (Pisum sativum). Front. Plant Sci. 2014, 5, 149. [Google Scholar] [CrossRef] [PubMed]
- Nable, R.; Loneragan, J. Translocation of manganese in subterranean clover (Trifolium subterraneum L. Cv. Seaton Park) I. redistribution during vegetative growth. Funct. Plant Biol. 1984, 11, 101–111. [Google Scholar]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Mauk, C.S.; Noodén, L.D. Regulation of mineral redistribution in pod-bearing soybean explants. J. Exp. Bot. 1992, 43, 1429–1440. [Google Scholar] [CrossRef]
- Hocking, P.J. Dry-matter production, mineral nutrient concentrations, and nutrient distribution and redistribution in irrigated spring wheat. J. Plant Nutr. 1994, 17, 1289–1308. [Google Scholar] [CrossRef]
- Gombert, J.; Etienne, P.; Ourry, A.; Le Dily, F. The expression patterns of SAG12/Cab genes reveal the spatial and temporal progression of leaf senescence in Brassica napus L. with sensitivity to the environment. J. Exp. Bot. 2006, 57, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Etienne, P.; Desclos, M.; Le Gou, L.; Gombert, J.; Bonnefoy, J.; Maurel, K.; Le Dily, F.; Ourry, A.; Avice, J.-C. N-protein mobilisation associated with the leaf senescence process in oilseed rape is concomitant with the disappearance of trypsin inhibitor activity. Funct. Plant Biol. 2007, 34, 895–906. [Google Scholar] [CrossRef]
- Desclos, M.; Etienne, P.; Coquet, L.; Jouenne, T.; Bonnefoy, J.; Segura, R.; Reze, S.; Ourry, A.; Avice, J.C. A combined 15N tracing/proteomics study in Brassica napus reveals the chronology of proteomics events associated with N remobilisation during leaf senescence induced by nitrate limitation or starvation. Proteom 2009, 9, 3580–3608. [Google Scholar] [CrossRef] [PubMed]
- Dubousset, L.; Abdallah, M.; Desfeux, A.; Etienne, P.; Meuriot, F.; Hawkesford, M.J.; Gombert, J.; Segura, R.; Bataille, M.-P.; Reze, S.; et al. Remobilization of leaf S compounds and senescence in response to restricted sulphate supply during the vegetative stage of oilseed rape are affected by mineral N availability. J. Exp. Bot. 2009, 60, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.P.; Graham, R.D. Distribution and remobilization of iron and copper in wheat. Ann. Bot. 2005, 95, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Baten, A.; Waters, D.L.E.; Pantoja, O.; Julia, C.C.; Wissuwa, M.; Heuer, S.; Kretzschmar, T.; Rose, T.J. Phosphorus remobilization from rice flag leaves during grain filling: An RNA-seq study. Plant Biotechnol. J. 2016, 15, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An overview of uptake, essentiality and toxicity in plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.S.; Gong, J.M. Vacuolar sequestration capacity and long-distance metal transport in plants. Front. Plant Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ricachenevsky, F.K.; Koprovski Menguer, P.; Sperotto, R.A. kNACking on heaven’s door: How important are NAC transcription factors for leaf senescence and Fe/Zn remobilization to seeds? Front. Plant Sci. 2013, 4, 226. [Google Scholar] [CrossRef] [PubMed]
- Hill, J. The remobilization of nutrients from leaves. J. Plant Nutr. 1980, 2, 407–444. [Google Scholar] [CrossRef]
- Sarda, X.; Diquelou, S.; Abdallah, M.; Nesi, N.; Cantat, O.; Le Gouee, P.; Avice, J.C.; Ourry, A. Assessment of sulphur deficiency in commercial oilseed rape crops from plant analysis. J. Agri. Sci. 2013, 1–18. [Google Scholar] [CrossRef]
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.C.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Song, H.X.; Liao, Q.; Yu, Y.; Jian, S.F.; Lepo, J.E.; Liu, Q.; Rong, X.M.; Tian, C.; Zeng, J.; et al. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 2016, 170, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol. 2008, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Schiltz, S.; Munier-Jolain, N.; Jeudy, C.; Burstin, J.; Salon, C. Dynamics of exogenous nitrogen partitioning and nitrogen remobilisation from vegetative organs in pea (Pisum sativum L.) revealed by 15N in vivo labelling throughout the seed filling. Plant Physiol. 2005, 137, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Galili, G.; Genschik, P.; Fernie, A.R.; Avin-Wittenberg, T. Autophagy in plants—What’s new on the menu? Trends Plant Sci. 2016, 21, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Wada, S.; Izumi, M.; Makino, A.; Ishida, H. Evidence for contribution of autophagy to Rubisco degradation during leaf senescence in Arabidopsis thaliana. Plant Cell Environ. 2013, 36, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Distelfeld, A.; Cakmak, I.; Peleg, Z.; Ozturk, L.; Yazici, A.M.; Budak, H.; Saranga, Y.; Fahima, T. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant. 2007, 129, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Uauay, C.; Brevis, J.C.; Dubcovsky, J. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Tabbita, F.; Cantu, D.; Buffalo, V.; Avni, R.; Vazquez-Gross, H.; Zhao, R.; Conley, C.J.; Distelfeld, A.; Dubcovksy, J. Regulation of Zn and Fe transporters by the GPC1 gene during early wheat monocarpic senescence. BMC Plant Biol. 2014, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; de Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume nodule senescence: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005, 165, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Guerra, J.C.P.; Keyser, A.D.; De Rycke, R.; Rombauts, S.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Holsters, M.; Goormachtig, S. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol. 2006, 141, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, J.W.; Harlow, C.; Theil, E.C. Evidence for reutilization of nodule iron in soybean seed development. J. Plant Nutr. 1998, 21, 913–927. [Google Scholar] [CrossRef]
- Hakoyama, T.; Watanabe, H.; Tomita, J.; Yamamoto, A.; Sato, S.; Mori, Y.; Kouchi, H.; Suganuma, N. Nicotianamine synthase specifically expressed in root nodules of Lotus japonicus. Planta 2009, 230, 309–317. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, M.; Matthiadis, A.; Sáez, Á.; Long, T.A. Fixating on metals: New insights into the role of metals in nodulation and symbiotic nitrogen fixation. Front. Plant Sci. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Ghandilyan, A.; Barboza, L.; Tisné, S.; Granier, C.; Reymond, M.; Koornneef, M.; Schat, H.; Aarts, M.G.M. Genetic analysis identifies quantitative trait loci controlling rosette mineral concentrations in Arabidopsis thaliana under drought. New Phytol. 2009, 184, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Gallardo, K.; Courty, P.-E.; Le Signor, C.; Wipf, D.; Vernoud, V. Sulfate transporters in the plant’s response to drought and salinity: Regulation and possible functions. Front. Plant Sci. 2014, 5, 580. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Jensen, C.R.; Mogensen, V.O.; Andersen, M.N.; Henson, I.E. Root signalling and osmotic adjustment during the intermittent soil drying sustain grain yields of field grown wheat. Field Crops Res. 1999, 62, 35–52. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ. 2003, 26, 1621–1631. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Vassileva, V.; Feller, U. Selection and breeding of suitable crop genotypes for drought and heat periods in a changing climate: Which morphological and physiological properties should be considered? Agriculture 2016, 6, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Lawlor, D.W. Genetic engineering to improve plant performance under drought: Physiological evaluation of achievements, limitations, and possibilities. J. Exp. Bot. 2013, 64, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Day, W.; Johnston, A.E.; Legg, B.J.; Parkinson, K.J. Growth of spring barley under drought: Crop development, photosynthesis, dry-matter accumulation and nutrient content. J. Agric. Sci. 1981, 96, 167–186. [Google Scholar] [CrossRef]
- Maleki Farahani, S.M.; Chaichi, M.R.; Mazaheri1, D.; Tavakkol Afshari, R.; Savaghebi, G.H. Barley grain mineral analysis as affected by different fertilizing systems and by drought stress. J. Agric. Sci. Technol. 2011, 13, 315–326. [Google Scholar]
- Guzmán, C.; Autrique, J.E.; Mondal, S.; Singh, R.P.; Govindan, V.; Morales-Dorantes, A.; Posadas-Romano, G.; Crossa, J.; Ammar, K.; Peña, R.J. Response to drought and heat stress on wheat quality, with special emphasis on bread-making quality, in durum wheat. Field Crops Res. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Haberle, J.; Svoboda, P.; Raimanová, I. The effect of post-anthesis water supply on grain nitrogen concentration and grain nitrogen yield of winter wheat. Plant Soil Environ. 2008, 7, 304–312. [Google Scholar]
- Rose, T.J.; Raymond, C.A.; Bloomfield, C.; King, G.J. Perturbation of nutrient source-sink relationships by post-anthesis stresses results in differential accumulation of nutrients in wheat grain. J. Plant Nutr. Soil Sci. 2015, 178, 89–98. [Google Scholar] [CrossRef]
- Zhao, C.-X.; He, M.-R.; Wang, Z.-L.; Wang, Y.-F.; Lin, Q. Effects of different water availability at post-anthesis stage on grain nutrition and quality in strong-gluten winter wheat. C. R. Biol. 2009, 332, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.G.; Fang, G.S.; San’an, N.; Ning, B.S.; He’ai, X.; Cheng, L.T. Differential responses of yield and selected nutritional compositions to drought stress in summer maize grains. J. Plant Nutr. 2010, 33, 1811–1818. [Google Scholar]
- Oktem, A. Effect of water shortage on yield, and protein and mineral compositions of drip-irrigated sweet corn in sustainable agricultural systems. Agric. Water Manag. 2008, 95, 1003–1010. [Google Scholar] [CrossRef]
- Bellaloui, N.; Gillen, A.M.; Mengistu, A.; Kebede, H.; Fisher, D.K.; Smith, J.R.; Reddy, K.N. Responses of nitrogen metabolism and seed nutrition to drought stress in soybean genotypes differing in slow-wilting phenotype. Front. Plant Sci. 2013, 4, 498. [Google Scholar] [CrossRef] [PubMed]
- Samarah, N.; Mullen, R.; Cianzio, S. Size distribution and mineral nutrients of soybean seeds in response to drought stress. J. Plant Nutr. 2004, 27, 815–835. [Google Scholar] [CrossRef]
- Kresović, B.; Gajic, B.A.; Tapanarova, A.; Dugalić, G. Yield and chemical composition of soybean seed under different irrigation regimes in the Vojvodina region. Plant Soil Environ. 2017, 63, 34–39. [Google Scholar]
- Nam, K.H.; Kim, D.Y.; Shin, H.J.; Nam, K.J.; An, J.H.; Pack, I.S.; Park, J.H.; Jeong, S.C.; Kim, H.B.; Kim, C.G. Drought stress-induced compositional changes in tolerant transgenic rice and its wild type. Food Chem. 2014, 153, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.S.; Del Peloso, M.J.; Bassinello, P.Z.; Guimarães, C.M.; Melo, L.C.; Faria, L.C. Genetic variability for iron and zinc content in common bean lines in interaction with water availability. Gen. Mol. Res. 2014, 13, 6773–6785. [Google Scholar] [CrossRef] [PubMed]
- Jacomini, E.; Bertani, A.; Mapelli, S. Accumulation of polyethylene glycol 6000 and its effects on water content and carbohydrate level in water-stressed tomato plants. Can. J. Bot. 1988, 66, 970–973. [Google Scholar] [CrossRef]
- Verslues, P.E.; Ober, E.S.; Sharp, R.E. Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol. 1998, 116, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Etienne, P.; Diquélou, S.; Trouverie, J.; Billard, V.; Yvin, J.C.; Ourry, A. Nutrient deficiencies modify the ionomic composition of plant tissues: A focus on cross-talk between molybdenum and other nutrients in Brassica napus. J. Exp. Bot. 2016, 67, 5631–5641. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Gamboa, L.M.; Liu, S.; Langley, E.; Campbell, Z.; Castro-Guerrero, N.; Mendoza-Cozatl, D.; Lorence, A. Moderate to severe water limitation differentially affects the phenome and ionome of Arabidopsis. Funct. Plant Biol. 2017, 44, 94–106. [Google Scholar] [CrossRef]
- Baxter, I.R.; Vitek, O.; Lahner, B.; Muthukumar, B.; Borghi, M.; Morrissey, J.; Guerinot, M.L.; Salt, D.E. The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc. Nat. Acad. Sci. USA 2008, 105, 12081–12086. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.P.; Huguet, T.; Grusak, M. A. Identification of QTL affecting seed mineral concentrations and content in the model legume Medicago truncatula. Theor. Appl. Gen. 2009, 119, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Rubio-Wilhelmi, M.M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Umnajkitikorn, K.; del Mar Rubio Wilhelmi, M.; Wright, M.; Wang, S.; Blumwald, E. Delaying chloroplast turnover increases water-deficit stress tolerance through the enhancement of nitrogen assimilation in rice. J. Exp. Bot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [PubMed]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bagayoko, M.; George, E.; Romheld, V.; Buerkert, A.B. Effects of mycorrhizae and phosphorus on growth and nutrient uptake of millet, cowpea and sorghum on a West African soil. J. Agric. Sci. 2000, 135, 399–407. [Google Scholar] [CrossRef]


| Species | Leaf Remobilization of Macronutrients | Leaf Remobilization of Micronutrients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | K | S | P | Mg | Ca | Mn | Cu | Mo | B | Ni | Fe | Zn | |
| Triticum aestivum | 93% 1 | 95% 1 | 94% 1 | 89% 1 | 89% 1 | 89% 1 | 43% 1 | 71% 1 | 79% 1 | 0 1 | 0 1 | 0 1 | 92% 1 |
| Hordeum vulgare | 88% 1 | 96% 1 | 93% 1 | 87% 1 | 96% 1 | 89% 1 | 70% 1 | 84% 1 | 0 1 | 0 1 | 0 1 | 0 1 | 70% 1 |
| Oryza sativa | - | 47% 4 | 20% 4 | - | 45% 4 | 33% 4 | 65% 4 | 40% 4 | 46% 4 | - | 0 4 | 20% 4 | 29% 4 |
| Zea mays | 39% 1 | 72% 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 59% 1 | 0 1 | 0 1 | 0 1 |
| Brassica napus | 51% 1 | 37% 1 | 54% 1 | 55% 1 | 36% 1 | 0 1 | 0 1 | 50% 1 | 0 1 | 0 1 | 91% 1 | 68% 1 | 0 1 |
| Arabidopsis thaliana | 88% 2 | 86% 2 | 65% 2 | 78% 2 | 0 2 | 0 2 | 0 2 | 54% 2 | 78% 2 | - | 0 2 | 55% 2 | 54% 2 |
| Glycine max | 60% 5 | 63% 5 | - | 67% 5 | 49% 5 | 59% 5 | 42% 5 | 60% 5 | 96% 5 | 45% 5 | - | 38% 5 | 48% 5 |
| Pisum sativum | 72% 1 | 43% 1 | 57% 1 | 81% 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 0 1 | 47% 1 |
| - | 50% 3 | 65% 3 | 82% 3 | 50% 3 | - | 21% 3 | 73% 3 | - | - | - | 70% 3 | 32% 3 | |
| Species | Drought | Increased Seed Nutrient Content | Decreased Seed Nutrient Content | Refs. |
|---|---|---|---|---|
| Hordeum vulgare | Severe | N | P, K, Mg | [76] |
| Moderate | No effect | No effect | [76] | |
| Severe | N, Zn, Mn | No effect | [77] | |
| Triticum turgidum | Severe | N, Fe, Zn | No effect | [78] |
| Moderate | No effect | No effect | [78] | |
| Triticum aestivum | Severe | N | Not quantified | [79] |
| Severe | (N not quantified), K, Ca | No effect | [80] | |
| Moderate | [80] | |||
| Moderate | P, Mg, Zn | K | [81] | |
| Severe | P, Ca, Mg, Zn | K | [81] | |
| Zea mays | Severe | N, Ca, Mg, Cu, N | P, K | [82] |
| Severe | N | Fe, Zn, Cu | [83] | |
| Moderate | Fe, Zn, Cu | [83] | ||
| Glycine max | Moderate | No effect | N, K, P, Ca, Fe | [84] |
| Severe | P, Ca, Mn, Zn, Mo | No effect | [85] | |
| Severe | Ca, Fe | K, P, Mn, Cu, Zn | [86] | |
| Moderate | Ca, K | P, Mn, Cu, Zn | [86] | |
| Oryza sativa | Severe | N | Cu, Fe | [87] |
| Phaseolus vulgaris | Moderate | Fe, Zn | [88] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and Micronutrient Storage in Plants and Their Remobilization When Facing Scarcity: The Case of Drought. Agriculture 2018, 8, 14. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture8010014
Etienne P, Diquelou S, Prudent M, Salon C, Maillard A, Ourry A. Macro and Micronutrient Storage in Plants and Their Remobilization When Facing Scarcity: The Case of Drought. Agriculture. 2018; 8(1):14. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture8010014
Chicago/Turabian StyleEtienne, Philippe, Sylvain Diquelou, Marion Prudent, Christophe Salon, Anne Maillard, and Alain Ourry. 2018. "Macro and Micronutrient Storage in Plants and Their Remobilization When Facing Scarcity: The Case of Drought" Agriculture 8, no. 1: 14. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture8010014