Ommatissus lybicus Infestation in Relation to Spatial Characteristics of Date Palm Plantations in Oman
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Infestation Data
2.3. Factor Data
2.4. Data Analysis
3. Results
3.1. Infestation
3.2. Results of Ordinary Least Squares (OLS) Regression
3.3. Results of Geographically Weighted Regression (GWR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Khatri, S.A.H. Biological, Ecological and Phylogenic Studies of Pseudoligosita babylonica Viggiani, a Native Egg Parasitoid of Dubas Bug Ommatissus lybicus De Bergevin, the Major Pest of Date Palm in the Sultanate of Oman. Ph.D. Thesis, University of Reading, Reading, UK, 2011. [Google Scholar]
- Elwan, A.; Al-Tamimi, S. Life Cycle of Dubas Bug Ommatissus binotatus lybicus De berg. (Homoptera: Tropiduchidae) in Sultanate of Oman. Egypt. J. Agric. Res. 1999, 77, 1547–1553. [Google Scholar]
- Mokhtar, A.M.; Nabhani, A.; Saif, S. Temperature-Dependent Development of Dubas Bug, Ommatissus lybicus (Hemiptera: Tropiduchidae), an endemic Pest of Date Palm, Phoenix dactylifera. Eur. J. Entomol. 2010, 107. [Google Scholar] [CrossRef]
- Shah, A.; Zia, A.; Rafi, M.A.; Mehmood, S.A.; Aslam, S.; Chaudhry, M.T. Quantification of Honeydew Production Caused by Dubas Bug on Three Date Palm Cultivars. J. Entomol. Zool. Stud. 2016, 4, 478–484. [Google Scholar]
- Kinawy, M. Pests of Date Palm and Dates in the Sultanate of Oman; Diwan’s Office for Royal Court Affairs: Muscat, Oman, 2005. [Google Scholar]
- Hussain, A.A. Biology and Control of The Dubas Bug, Ommatissus binotatus lybicus De Berg. (Homoptera, Tropiduchidae), Infesting Date Palms in Iraq. Bull. Entomol. Res. 1963, 53, 737–745. [Google Scholar] [CrossRef]
- Howard, F. Hemiptera: Sternorrhyncha; CABI: Wallingford, UK, 2001; pp. 161–227. ISBN 085199 3265. [Google Scholar]
- El-Wakeil, N.; Gaafar, N.; Sallam, A.; Volkmar, C. Side Effects of Insecticides on Natural Enemies and Possibility of Their Integration in Plant Protection Strategies. In Insecticides-Development of Safer and More Effective Technologies; InTech: London, UK, 2013. [Google Scholar] [Green Version]
- Johnson, C.A.; Coutinho, R.M.; Berlin, E.; Dolphin, K.E.; Heyer, J.; Kim, B.; Leung, A.; Sabellon, J.L.; Amarasekare, P. Effects of Temperature and Resource Variation on Insect Population Dynamics: The Bordered Plant Bug as a Case Study. Funct. Ecol. 2016, 30, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Javed, M.; Sohail, M.; Sagheer, M. Environmental Effects on Insects and their Population Dynamics. J. Entomol. Zool. Stud. 2014, 2, 1–7. [Google Scholar]
- Altieri, M.; Nicholls, C. Biodiversity and Pest Management in Agroecosystems; CRC Press: London, UK, 2004; ISBN 148227793X. [Google Scholar]
- Smith, H.A.; McSorley, R. Intercropping and Pest Management: A Review of Major Concepts. Am. Entomol. 2000, 46, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Campos, R.I.; Vasconcelos, H.L.; Ribeiro, S.P.; Neves, F.S.; Soares, J.P. Relationship Between Tree Size and Insect Assemblages Associated with Anadenanthera macrocarpa. Ecography 2006, 29, 442–450. [Google Scholar] [CrossRef]
- Kautz, M.; Imron, M.A.; Dworschak, K.; Schopf, R. Dispersal variability and associated population-level consequences in tree-killing bark beetles. Mov. Ecol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Foster, J.R.; Townsend, P.A.; Mladenoff, D.J. Spatial Dynamics of a Gypsy Moth Defoliation Outbreak and Dependence on Habitat Characteristics. Landsc. Ecol. 2013, 28, 1307–1320. [Google Scholar] [CrossRef]
- Gurr, G.M.; Liu, J.; Johnson, A.C.; Woruba, D.N.; Kirchhof, G.; Fujinuma, R.; Sirabis, W.; Jeffery, Y.; Akkinapally, R. Pests, Diseases and Crop Protection Practices in The Smallholder Sweetpotato Production System of the Highlands of Papua New Guinea. PeerJ 2016, 4, e2703. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, A.; Ezeh, A. Effect of Variety and Nutrient on Insect Pest Infestation of Amaranthus spp. J. Appl. Sci. Environ. Manag. 2015, 19, 251–256. [Google Scholar] [CrossRef]
- Hartzell, F. Relation of Shelter to Abundance of Pear Midge. J. Econ. Entomol. 1932, 25, 351–355. [Google Scholar] [CrossRef]
- Johnson, C. Infestation of a Bean Field by Aphis fabae Scop. in Relation to Wind Direction. Ann. Appl. Biol. 1950, 37, 441–450. [Google Scholar] [CrossRef]
- Harcourt, D. Spatial Pattern of the Imported Cabbageworm, Pieris rapae (L.) (Lepidoptera: Pieridae), on Cultivated Cruciferae. Can. Entomol. 1961, 93, 945–952. [Google Scholar] [CrossRef]
- Nguyen, H.D.D.; Nansen, C. Edge-Biased Distributions of Insects. A Review. Agron. Sustain. Dev. 2018, 38, 11. [Google Scholar] [CrossRef]
- Al-Kindi, K.M.; Kwan, P.; Andrew, N.; Welch, M. Impact of Environmental Variables on Dubas Bug Infestation Rate: A Case Study from the Sultanate of Oman. PLoS ONE 2017, 12, e0178109. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sahragard, A.; Pezhman, H.; Ghadamyari, M. Effect of Climatic and Management Factors on the Abundance of Dubas Bug, Ommatissus Lybicus Bergevin (Hem.: Tropiduchidae) in Northern Hormozgan and Southern Fars Provinces. Plant Pests Res. 2013, 3, 63–67. [Google Scholar]
- Al-Kindi, K.M.; Kwan, P.; Andrew, N.R.; Welch, M. Impacts of Human-Related Practices on Ommatissus lybicus Infestations of Date Palm in Oman. PLoS ONE 2017, 12, e0171103. [Google Scholar] [CrossRef]
- Al Shidi, R.; Kumar, L.; Al-Khatri, S.; Alaufi, M.; Albahri, M. Does Solar Radiation Affect the Distribution of Dubas bug (Ommatissus lybicus de Bergevin) Infestation. Agriculture 2018, 8, 107. [Google Scholar] [CrossRef]
- Al Shidi, R.; Kumar, L.; Al-Khatri, S.; Albahri, M.; Alaufi, M. Relationship of Date Palm Tree Density to Dubas bug Ommatissus lybicus Infestation in Omani Orchards. Agriculture 2018, 8, 64. [Google Scholar] [CrossRef]
- Al Shidi, R.; Kumar, L.; Al-Khatri, S.; Al Ajmi, N. Temperature and Humidity Vertical Profiles in Date Palm Fields and its Effect on Dubas Bug (Ommatissus lybicus de Bergevin) Infestation Levels. unpublished.
- Klok, C.J.; Sinclair, B.J.; Chown, S.L. Upper Thermal Tolerance and Oxygen Limitation in Terrestrial Arthropods. J. Exp. Biol. 2004, 207, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, A.; AI-Mjeni, A. A Novel Approach to Determine the Efficacy of Control Measures Against Dubas Bug, Ommatissus lybicus de Berg, on Date Palms. JAMS 1999, 4, 1–4. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, J.; Wang, Y.; Liu, J.; Li, S. Exploring Spatially Variable Relationships Between NDVI and climatic Factors in a Transition Zone Using Geographically Weighted Regression. Theor. Appl. Climatol. 2015, 120, 507–519. [Google Scholar] [CrossRef]
- Shrestha, P.M. Comparison of Ordinary Least Square Regression, Spatial Autoregression, and Geographically Weighted Regression for Modeling Forest Structural Attributes Using a Geographical Information System (GIS)/Remote Sensing (RS) Approach. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2006. [Google Scholar]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression; John Wiley & Sons: Chichester, UK, 2003; ISBN 978-0-471-49616-8. [Google Scholar]
- Chen, G.; Zhao, K.; McDermid, G.J.; Hay, G.J. The Influence of Sampling Density on Geographically Weighted Regression: A Case Study Using Forest Canopy Height and Optical Data. Int. J. Remote Sens. 2012, 33, 2909–2924. [Google Scholar] [CrossRef]
- Sisterson, M.S.; Dwyer, D.P.; Uchima, S.Y. Alfalfa and Pastures: Sources of Pests or Generalist Natural Enemies? Environ. Entomol. 2018, 47, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.N.; Al-Lawatiya, M.H.; Al-Sharaqi, A.M. Biological Control of Some Important Insect Pests; Ministry of Agriculture and Fisheries: Muscat, Oman, 1994; pp. 325–341.
- Segoli, M.; Rosenheim, J.A. Should Increasing the Field Size of Monocultural Crops be Expected to Exacerbate Pest Damage? Agric. Ecosyst. Environ. 2012, 150, 38–44. [Google Scholar] [CrossRef]
- Sujayanand, G.; Sharma, R.; Shankarganesh, K.; Saha, S.; Tomar, R. Crop Diversification for Sustainable Insect Pest Management in Eggplant (Solanales: Solanaceae). Fla. Entomol. 2015, 305–314. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Corley, J.C. Spatial Pattern of Attacks of the Invasive Woodwasp Sirex noctilio, at Landscape and Stand Scales. PLoS ONE 2015, 10, e0127099. [Google Scholar] [CrossRef]
- Santos de Araújo, W.; Rebouças Julião, G.; Araújo Ribeiro, B.; Portes Abraham Silva, I.; Baptista dos Santos, B. Diversity of Galling Insects in Styrax pohlii (Styracaceae): Edge Effect and Use as Bioindicators. Rev. Biol. Trop. 2011, 59, 1589–1597. [Google Scholar] [CrossRef]
- Straw, N.; Ludlow, A. Small-Scale Dynamics and Insect Diversity on Plants. Oikos 1994, 71, 188–192. [Google Scholar] [CrossRef]
- Latifian, M.; Rahnama, A.A.; Sharifnezhad, H. Effects of Planting Pattern on Major Date Palm Pests and Diseases Injury Severity. Int. J. Agric. Crop Sci. 2012, 4, 1443–1451. [Google Scholar]
- Han, P. Bottom-up Effects of Fertilization and Irrigation on Plant-Herbivorous Insect-Natural Enemy Tri-Trophic Interactions in Agroecosystems. Ph.D. Thesis, Université Nice Sophia Antipolis, Nice, France, 2014. [Google Scholar]
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 2, ISBN 0585304556. [Google Scholar]
- Scriber, J.M. Host-Plant Suitability. In Chemical Ecology of Insects; Springer: Berlin/Heidelberg, Germany, 1984; pp. 159–202. [Google Scholar]
- Wajnberg, E.; Colazza, S. Chemical Ecology of Insect Parasitoids; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1118409604. [Google Scholar]
- Mannan, M.; Tarannum, N. Infestation of Four Mustard Varieties by Lipaphis erysimi (Kalt) in Relation to Different Levels of Irrigation. Bangladesh J. Agric. Res. 2016, 41, 625–632. [Google Scholar] [CrossRef]
- Huberty, A.F.; Denno, R.F. Plant Water Stress and its Consequences for Herbivorous Insects: A New Synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Faulkenberry, M.; Hedden, R.; Culin, J. Hemlock Susceptibility to Hemlock Woolly Adelgid Attack in the Chattooga River Watershed. Southeast. Nat. 2009, 129–140. [Google Scholar] [CrossRef]
- Shah, A.; Mohsin, A.U.; Hafeez, Z.; Naeem, M.; Haq, M.I.U. Eggs Distribution Behaviour of Dubas bug (Ommatissus lybicus: Homoptera: Tropiduchidae) in relation to Seasons and Some Physico-Morphic Characters of Date Palm Leaves. J. Insect Behav. 2013, 26, 371–386. [Google Scholar] [CrossRef]
- Edmunds, G.F.; Alstad, D.N. Responses of Black Pineleaf Scales to Host Plant Variability. In Insect Life History Patterns; Springer: Berlin/Heidelberg, Germany, 1981; pp. 29–38. [Google Scholar]
- Régolini, M.; Castagneyrol, B.; Dulaurent-Mercadal, A.-M.; Piou, D.; Samalens, J.-C.; Jactel, H. Effect of Host Tree Density and Apparency on The Probability of Attack by the Pine Processionary Moth. For. Ecol. Manag. 2014, 334, 185–192. [Google Scholar] [CrossRef]
- Macfadyen, S.; Muller, W. Edges in Agricultural Landscapes: Species Interactions and Movement of Natural Enemies. PLoS ONE 2013, 8, e59659. [Google Scholar] [CrossRef]
- Akkuzu, E.; Dingiloglu, E.; Unal, S. Edge Effects on Gall-Inducing Insect Mikiola Fagi (Diptera: Cecidomyiidae) in the Oriental Beach Forests. Pak. J. Zool. 2015, 47, 685–690. [Google Scholar]
- Free, J.; Williams, I.H. A Survey of the Damage Caused to Crops of Oil-Seed Rape (Brassica napus L.) by Insect Pests in South-Central England and their Effect on Seed Yield. J. Agric. Sci. 1978, 90, 417–424. [Google Scholar] [CrossRef]
- Schneider, G.; Krauss, J.; Riedinger, V.; Holzschuh, A.; Steffan-Dewenter, I. Biological Pest Control and Yields Depend on Spatial and Temporal Crop Cover Dynamics. J. Appl. Ecol. 2015, 52, 1283–1292. [Google Scholar] [CrossRef]
- Keren, I.N.; Menalled, F.D.; Weaver, D.K.; Robison-Cox, J.F. Interacting Agricultural Pests and Their Effect on Crop Yield: Application of A Bayesian Decision Theory Approach to the Joint Management of Bromus tectorum and Cephus cinctus. PLoS ONE 2015, 10, e0118111. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, T. Ecological Strategies of Forest Insects: The Need for a Community-Level Approach to Reforestation. New For. 1986, 1, 57–66. [Google Scholar] [CrossRef]





| Serial Number | Factors | Description |
|---|---|---|
| 1 | Planting pattern | Regular = 1, semi-regular = 2 and random = 3. |
| 2 | Irrigation Interval | Number of days between irrigation cycles. |
| 3 | Landform index | Increases as the slope increases. |
| 4 | Side growing area | Presence of side growing area (0 = nil, 1 = minor area, 2 = clearly distinguishable area form the image, 3 = almost 20% of total area, 4 = almost 40% of total area and 5 = nearly 50% of total area. |
| 5 | Location area | In square meters |
| 6 | Distance to nearest date palm plantation | In meters |
| 7 | Tree distance to edge of field | In meters |
| 8 | Surrounding tree height | Graded as 0, when none of the surrounding trees were graded as high; 1, when at least one of the surrounding trees was graded as high; 2, when two of the surrounding trees were graded as high and one as medium; 3, when three of the surrounding trees were graded as high or when two were graded as high and two as medium; 4, when all four of the surrounding trees were graded as high. |
| Variable | Four Seasons | Autumn | Spring | |||
|---|---|---|---|---|---|---|
| Coefficient | VIF | Coefficient | VIF | Coefficient | VIF | |
| Intercept | 0.617 * | --- | 0.415 ** | ---- | 1.827 ** | ---- |
| Planting pattern | 0.462 * | 1.40 | 0.311 ** | 1.40 | 1.187 ** | 1.40 |
| Irrigation Interval | 0.055 * | 1.62 | 0.061 ** | 1.76 | 0.025 | 1.76 |
| Landform index | 0.092 * | 1.40 | 0.266 ** | 1.40 | 0.044 | 1.40 |
| Side growing area | −0.226 * | 1.20 | −0.166 ** | 1.21 | −0.512 ** | 1.21 |
| Field area | −0.000 * | 1.46 | −0.000 ** | 1.46 | −0.000 ** | 1.46 |
| Near village | −0.000 * | 1.26 | −0.000 * | 1.35 | −0.000 ** | 1.35 |
| Near edge | 0.000 * | 1.10 | 0.000 | 1.12 | 0.000 ** | 1.12 |
| Near trees height | 0.074 * | 1.13 | 0.014 | 1.16 | 0.284 ** | 1.16 |
| Parameter | Four Seasons | Autumn | Spring |
|---|---|---|---|
| Number of observations | 399 | 399 | 399 |
| Multiple R2 | 0.50 | 0.42 | 0.42 |
| Joint F-statistic | 48.79 | 31.34 | 31.82 |
| Joint Wald statistic | 808.29 | 753.41 | 588.52 |
| Koenker (BP *) statistic | 32.93 | 42.80 | 73.38 |
| Jarque-Bera statistic | 6.42 | 4.16 | 14.64 |
| Akaike information criterion (AICc) | 790.72 | 828.72 | 1527.62 |
| Variable Name | Four Seasons | Autumn | Spring |
|---|---|---|---|
| Bandwidth | 36,035.46 | 35,012.06 | 20,004.21 |
| Residual squares | 126.50 | 145.94 | 735.11 |
| Effective number | 17.41 | 14.06 | 23.89 |
| Sigma | 0.58 | 0.62 | 1.40 |
| AICc | 706.86 | 758.00 | 1421.77 |
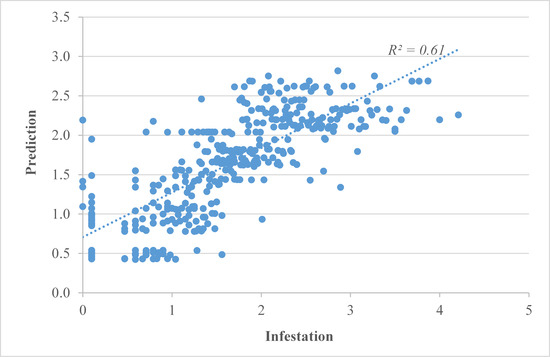
| R2 | 0.61 | 0.52 | 0.58 |
| R2 adjusted | 0.59 | 0.50 | 0.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Shidi, R.H.; Kumar, L.; Al-Khatri, S.A.H.; Al-Ajmi, N.A. Ommatissus lybicus Infestation in Relation to Spatial Characteristics of Date Palm Plantations in Oman. Agriculture 2019, 9, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture9030050
Al Shidi RH, Kumar L, Al-Khatri SAH, Al-Ajmi NA. Ommatissus lybicus Infestation in Relation to Spatial Characteristics of Date Palm Plantations in Oman. Agriculture. 2019; 9(3):50. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture9030050
Chicago/Turabian StyleAl Shidi, Rashid H., Lalit Kumar, Salim A. H. Al-Khatri, and Najat A. Al-Ajmi. 2019. "Ommatissus lybicus Infestation in Relation to Spatial Characteristics of Date Palm Plantations in Oman" Agriculture 9, no. 3: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture9030050