Can Hairy Vetch Cover Crop Affects Arsenic Accumulation in Vegetable Crops?
Abstract
:1. Introduction
2. Materials and Methods
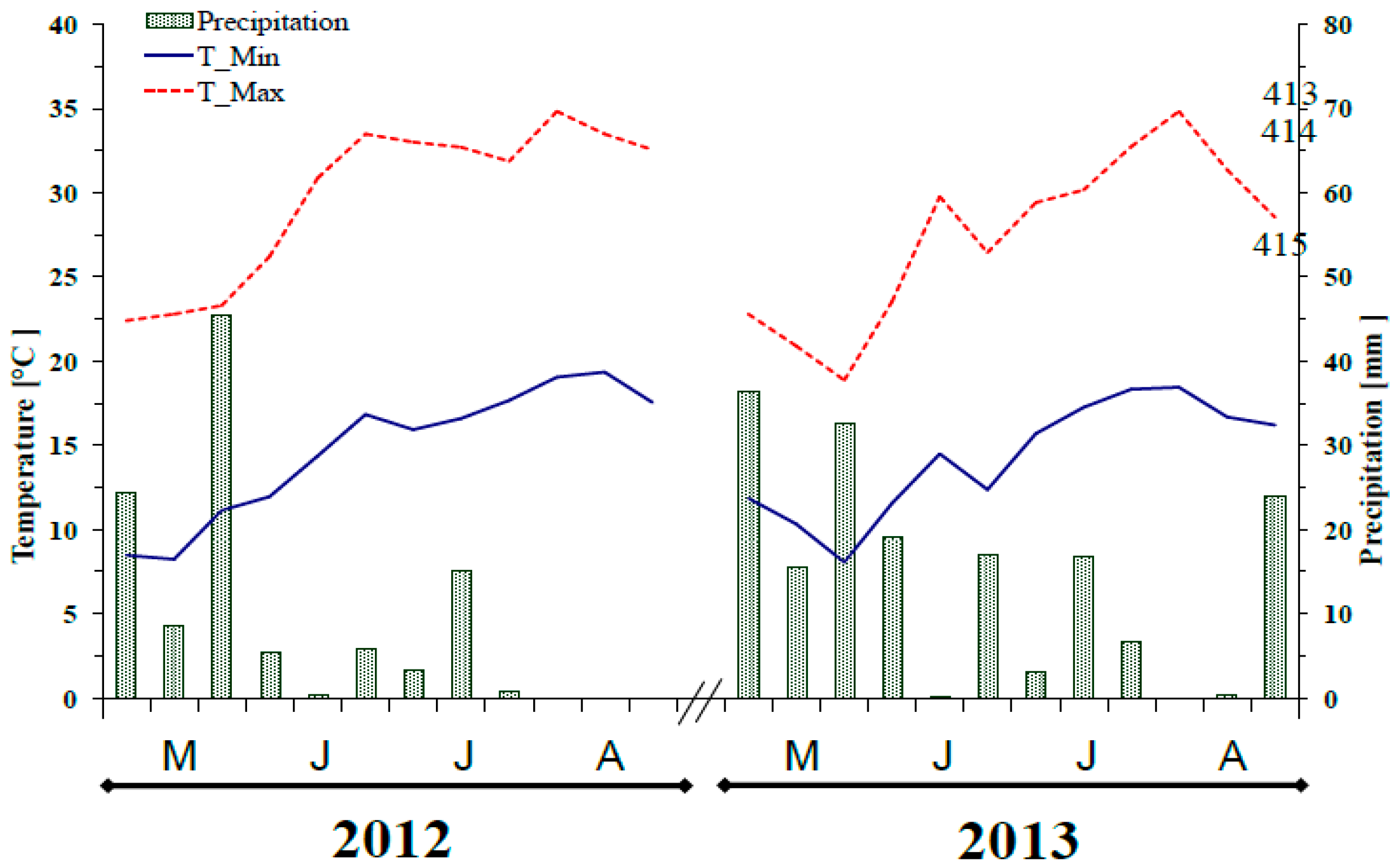
2.1. Study Area and Soil Characteristics
2.2. Experimental Design and Field Description
2.3. Sampling and Analysis of Soil, Hairy Vetch and Vegetable Crops
2.4. Data Analysis and Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lynch, H.N.; Greenberg, G.I.; Pollock, M.C.; Lewis, A.S. A comprehensive evaluation of inorganic arsenic in food and considerations for dietary intake analyses. Sci. Total Environ. 2014, 496, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Stazi, S.R.; Mancinelli, R.; Marabottini, R.; Allevato, E.; Radicetti, E.; Campiglia, E.; Marinari, S. Influence of organic management on As bioavailability: Soil quality and tomato As uptake. Chemosphere 2018, 211, 352–359. [Google Scholar] [CrossRef]
- Koch, I.; Dee, J.; House, K.; Sui, J.; Zhang, J.; McKnight-Whitford, A.; Reimer, K.J. Bioaccessibility and speciation of arsenic in country foods from contaminated sites in Canada. Sci. Total Environ. 2013, 449, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Li, C.; Gaudin, A.C.M.; Akbar, N.; Carrijo, D.R.; Reis, A.F.B.; Linquist, B.A.; Parikh, S.J. Impacts of variable soil drying in alternate wetting and drying rice systems on yields, grain arsenic concentration and soil moisture dynamics. Field Crop. Res. 2018, 222, 101–110. [Google Scholar]
- Shaw, R.K.; Maddaloni, M.; Deeb, M.; Cheng, Z.; Groffman, P.M.; Paltseva, A. Accumulation of arsenic and lead in garden-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640–641, 273–283. [Google Scholar]
- Marin, A.R.; Masscheleyn, P.H.; Patrick, W.H. The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant Soil 1992, 139, 175–183. [Google Scholar] [CrossRef]
- Burló, F.; Guijarro, I.; Carbonell-Barrachina, A.A.; Valero, D.; Martínez-Sánchez, F. Arsenic species: Effects on and accumulation by tomato plants. J. Agric. Food Chem. 1999, 47, 1247–1253. [Google Scholar] [CrossRef]
- Madeira, A.C.; de Varennes, A.; Abreu, M.M.; Esteves, C.; Magalhães, M.C.F. Tomato and parsley growth, arsenic uptake and translocation in a contaminated amended soil. J. Geochem. Explor. 2012, 123, 114–121. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Tittonell, P. Ecological intensification of agriculture-sustainable by nature. Curr. Opin. Environ. Sustain. 2014, 8, 53–61. [Google Scholar] [CrossRef]
- Alberola, C.; Lichtfouse, E.; Navarrete, M.; Debaeke, P.; Souchère, V. Agronomy for sustainable development. Italy J. Agron. 2008, 3, 77–78. [Google Scholar]
- Hobbs, P.R. Conservation agriculture: What is it and why is it important for future sustainable food production? J. Agric. Sci. 2007, 145, 127–137. [Google Scholar] [CrossRef]
- Komatsuzaki, M.; Ohta, H. Soil management practices for sustainable agro-ecosystems. Sustain. Sci. 2007, 2, 103–120. [Google Scholar] [CrossRef]
- Hartwig, N.L.; Ammon, H.U. Cover crops and living mulches. Weed Sci. 2002, 50, 688–699. [Google Scholar] [CrossRef]
- Radicetti, E.; Campiglia, E.; Marucci, A.; Mancinelli, R. How winter cover crops and tillage intensities affect nitrogen availability in eggplant. Nutr. Cycl. Agroecosyst. 2017, 108, 177–194. [Google Scholar] [CrossRef]
- Radicetti, E.; Mancinelli, R.; Moscetti, R.; Campiglia, E. Management of winter cover crop residues under different tillage conditions affects nitrogen utilization efficiency and yield of eggplant (Solanum melanogena L.) in Mediterranean environment. Soil Tillage Res. 2016, 155, 329–338. [Google Scholar] [CrossRef]
- Snapp, S.; Swinton, S.; Labarta, R. Evaluating cover crops for benefits, costs and performance within cropping system niches. Agron. J. 2005, 322–332. [Google Scholar]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J.; et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Robačer, M.; Canali, S.; Kristensen, H.L.; Bavec, F.; Mlakar, S.G.; Jakop, M.; Bavec, M. Cover crops in organic field vegetable production. Sci. Hortic. 2016, 208, 104–110. [Google Scholar] [CrossRef]
- Radicetti, E.; Mancinelli, R.; Campiglia, E. Impact of managing cover crop residues on the floristic composition and species diversity of the weed community of pepper crop (Capsicum annuum L.). Crop Prot. 2013, 44, 109–119. [Google Scholar] [CrossRef]
- Kramberger, B.; Gselman, A.; Janzekovic, M.; Kaligaric, M.; Bracko, B. Effects of cover crops on soil mineral nitrogen and on the yield and nitrogen content of maize. Eur. J. Agron. 2009, 31, 103–109. [Google Scholar] [CrossRef]
- Mafakheri, S.; Ardakani, M.R.; Meighani, F.; Mirhadi, M.J.; Vazan, S. Rye cover crop management affects weeds and yield of corn (Zea mays L.). Not. Bot. Hort. Agrobot. 2010, 38, 117–123. [Google Scholar]
- Sainju, U.M.; Whitehead, W.F.; Singh, B.P.; Wang, S. Tillage, cover crops, and nitrogen fertilization effects on soil nitrogen and cotton and sorghum yields. Eur. J. Agron. 2006, 25, 372–382. [Google Scholar] [CrossRef]
- Radicetti, E.; Osipitan, O.A.; Reza, A.; Langeroodi, S.; Marinari, S.; Mancinelli, R. CO2 Flux and C Balance due to the Replacement of Bare Soil with Agro-Ecological Service Crops in Mediterranean Environment. Agriculture 2019, 9, 71. [Google Scholar] [CrossRef]
- Langeroodi, A.S.; Radicetti, E.; Campiglia, E. How cover crop residue management and herbicide rate affect weed management and yield of tomato (Solanum lycopersicon L.) crop. Renew. Agric. Food Syst. 2018, 1–9. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Cover crops and mulches influence weed management and weed flora composition in strip-tilled tomato (Solanum lycopersicum). Weed Res. 2015, 55, 416–425. [Google Scholar] [CrossRef]
- Soil Survey Staff Soil Survey Geographic (SSURGO) Database for [U.S.]. Available online: http://soildatamart.nrcs.usda.gov (accessed on 11 April 2019).
- Campiglia, E.; Caporali, F.; Radicetti, E.; Mancinelli, R. Hairy vetch (Vicia villosa Roth.) cover crop residue management for improving weed control and yield in no-tillage tomato (Lycopersicon esculentum Mill.) production. Eur. J. Agron. 2010, 33, 94–102. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Papetti, P.; Rossi, G. Heavy metals in the fishery products of low Lazio and the use of metallothionein as a biomarker of contamination. Environ. Monit. Assess. 2009, 159, 589–598. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. SAS System for Mixed Models; SAS Institute: Cary, NC, USA, 1996; ISBN 1555447791. [Google Scholar]
- Cody, R.P.; Smith, J.K. Applied Statistics and the SAS Programming Language, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Agehara, S.; Warncke, D.D. Soil Moisture and Temperature Effects on Nitrogen Release from Organic Nitrogen Sources. Soil Sci. Soc. Am. J. 2005, 69, 1844–1855. [Google Scholar] [CrossRef]
- Steenwerth, K.; Belina, K.M. Cover crops enhance soil organic matter, carbon dynamics and microbiological function in a vineyard agroecosystem. Appl. Soil Ecol. 2008, 40, 359–369. [Google Scholar] [CrossRef]
- Kong, A.Y.Y.; Six, J. Microbial community assimilation of cover crop rhizodeposition within soil microenvironments in alternative and conventional cropping systems. Plant Soil 2012, 356, 315–330. [Google Scholar] [CrossRef]
- Radicetti, E.; Massantini, R.; Campiglia, E.; Mancinelli, R.; Ferri, S.; Moscetti, R. Yield and quality of eggplant (Solanum melongena L.) as affected by cover crop species and residue management. Sci. Hortic. 2016, 204, 161–171. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Brunetti, P.; Mancinelli, R. Do cover crop species and residue management play a leading role in pepper productivity? Sci. Hortic. 2014, 166, 97–104. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific report of EFSA—Dietary exposure to inorganic arsenic in the Europe population. EFSA J. 2014, 12, 3597. [Google Scholar]
- De Oliveira, L.M.; Suchismita, D.; Gress, J.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic uptake by lettuce from As-contaminated soil remediated with Pteris vittata and organic amendment. Chemosphere 2017, 176, 249–254. [Google Scholar] [CrossRef]
| Hairy Vetch | Transplanting | Harvesting | ||
|---|---|---|---|---|
| Aboveground biomass (g m−2d.w.) | 564 a | (45) | 214 b | (28) |
| Carbon in biomass (%) | 41.5 a | (0.22) | 39.8 b | (1.09) |
| Nitrogen in biomass (%) | 3.8 a | (0.20) | 2.7 b | (0.02) |
| C:N in biomass | 11.1 b | (0.7) | 14.7 a | (0.5) |
| Carbon in biomass (kg C ha−1) | 2337 a | (176) | 848 b | (94) |
| Nitrogen in biomass (kg N ha−1) | 214 a | (26) | 58 b | (8) |
| TOC (%) | TON (%) | Cmic (g Cmic g−1) | Nmic (g Nmic g−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Conventional | 1.521 b | (0.01) | 0.135 b | (0.01) | 263.33 b | (11.80) | 111.51 a | (7.79) |
| Hairy vetch | 1.793 a | (0.12) | 0.161 a | (0.01) | 326.39 a | (21.15) | 110.19 a | (0.77) |
| Fruits Production | Leaves | Stems | Roots | HI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ---- (g m−2 of f.w.) ---- | ------------------- (g m−2 of d.w.) ----------------- | |||||||||
| Tomato | ||||||||||
| Conventional | 5573.9 b | (152.9) | 176.9 b | (10.4) | 317.0 b | (7.6) | 55.0 b | (4.8) | 0.72 b | (0.01) |
| Hairy vetch | 7016.7 a | (143.0) | 212.6 a | (7.6) | 382.2 a | (8.6) | 70.3 a | (3.3) | 0.86 a | (0.03) |
| Sweet pepper | ||||||||||
| Conventional | 5218.3 b | (1133.7) | 163.8 b | (6.3) | 205.9 b | (6.3) | 48.9 b | (3.4) | 0.95 a | (0.03) |
| Hairy vetch | 7448.3 a | (287.4) | 248.4 a | (1.5) | 248.4 a | (1.5) | 70.1 a | (1.6) | 1.05 a | (0.20) |
| Zucchini | ||||||||||
| Conventional | 7286.4 b | (183.6) | 73.2 b | (5.6) | 92.9 b | (8.5) | 7.0 a | (0.9) | 1.57 b | (0.04) |
| Hairy vetch | 8147.6 a | (297.8) | 93.3 a | (7.1) | 134.0 a | (7.1) | 9.2 a | (0.7) | 2.37 a | (0.07) |
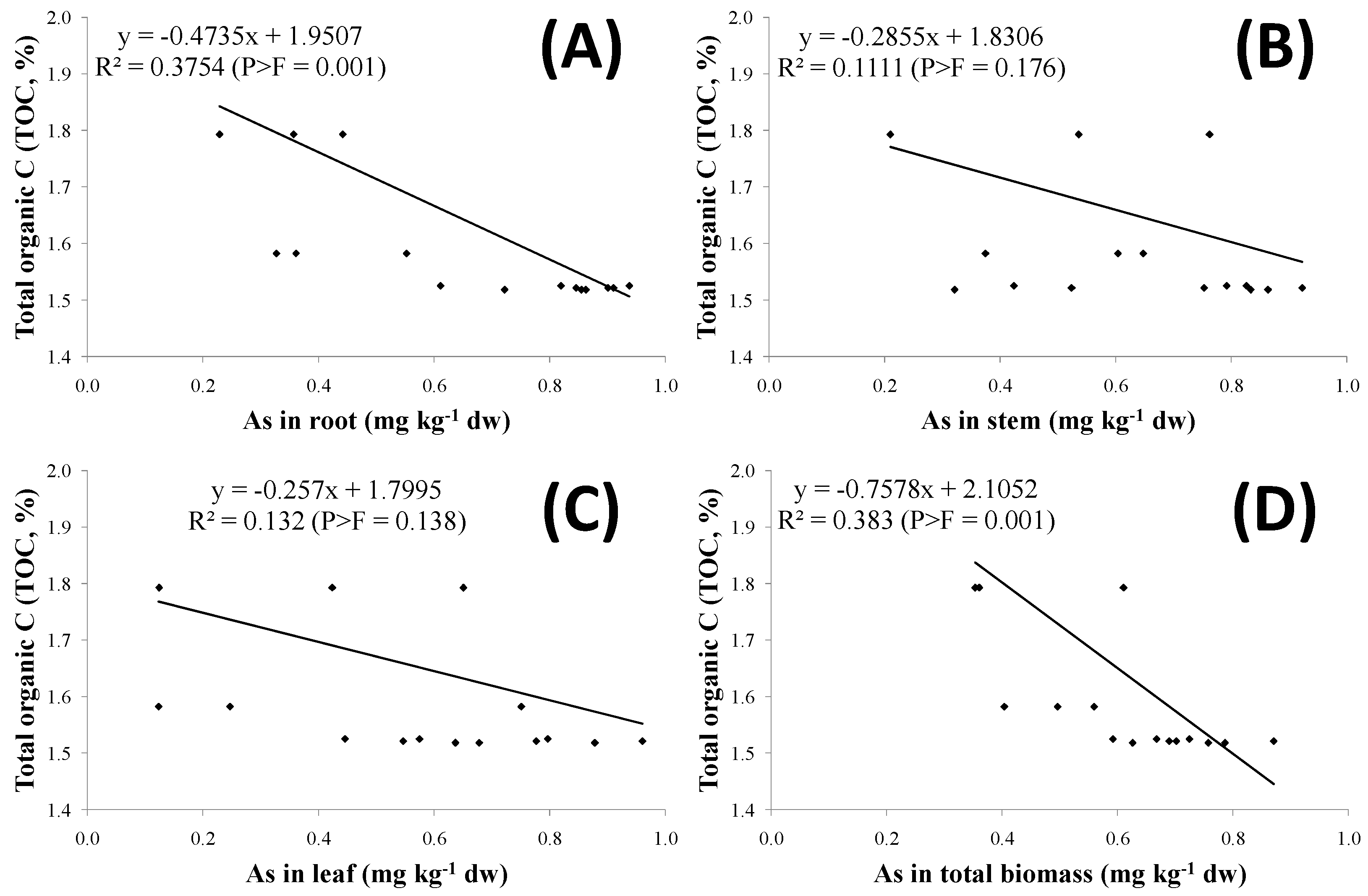
| As in Fruits | As in Root | As in Stem | As in Leaves | As in Biomass | As in Biomass | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| -------------------------------------------- mg kg−1 of d.w ---------------------------------------------- | mg m−2 | ||||||||||
| Tomato | |||||||||||
| Conventional | 0.075 a (0.004) | 0.726 a | (0.068) | 0.871 a | (0.028) | 0.677 a | (0.058) | 0.79 a | (0.04) | 434.3 a | (12.3) |
| Hairy vetch | 0.065 a (0.003) | 0.360 b | (0.002) | 0.718 a | (0.035) | 0.340 b | (0.051) | 0.56 b | (0.03) | 372.2 b | (24.4) |
| Sweet pepper | |||||||||||
| Conventional | 0.084 a (0.004) | 0.862 a | (0.027) | 0.793 a | (0.023) | 0.543 a | (0.055) | 0.70 a | (0.03) | 295.8 a | (22.4) |
| Hairy vetch | 0.078 a (0.004) | 0.546 b | (0.058) | 0.550 b | (0.028) | 0.145 b | (0.022) | 0.38 b | (0.02) | 206.3 b | (7.8) |
| Zucchini | |||||||||||
| Conventional | 0.070 a (0.003) | 0.901 a | (0.022) | 0.423 a | (0.059) | 0.879 a | (0.047) | 0.64 a | (0.03) | 110.8 a | (6.0) |
| Hairy vetch | 0.065 a (0.003) | 0.325 b | (0.055 | 0.292 b | (0.048) | 0.741 b | (0.049) | 0.46 b | (0.06) | 111.2 a | (18.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancinelli, R.; Radicetti, E.; Muleo, R.; Marinari, S.; Bravo, I.; Papetti, P. Can Hairy Vetch Cover Crop Affects Arsenic Accumulation in Vegetable Crops? Agriculture 2019, 9, 89. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture9050089
Mancinelli R, Radicetti E, Muleo R, Marinari S, Bravo I, Papetti P. Can Hairy Vetch Cover Crop Affects Arsenic Accumulation in Vegetable Crops? Agriculture. 2019; 9(5):89. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture9050089
Chicago/Turabian StyleMancinelli, Roberto, Emanuele Radicetti, Rosario Muleo, Sara Marinari, Ilenia Bravo, and Patrizia Papetti. 2019. "Can Hairy Vetch Cover Crop Affects Arsenic Accumulation in Vegetable Crops?" Agriculture 9, no. 5: 89. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture9050089







