Sex Similarities and Differences in Intelligence in Children Aged Two to Eight: Analysis of SON-R 2–8 Scores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. SON-R 2–8 Subtests
2.3.1. PS IQ (Gv) Subtests
Puzzles
Patterns
Mosaics
2.3.2. RS IQ (Gf) Subtests
Categories
Situations
Analogies
2.4. Procedure and Statistical Analysis
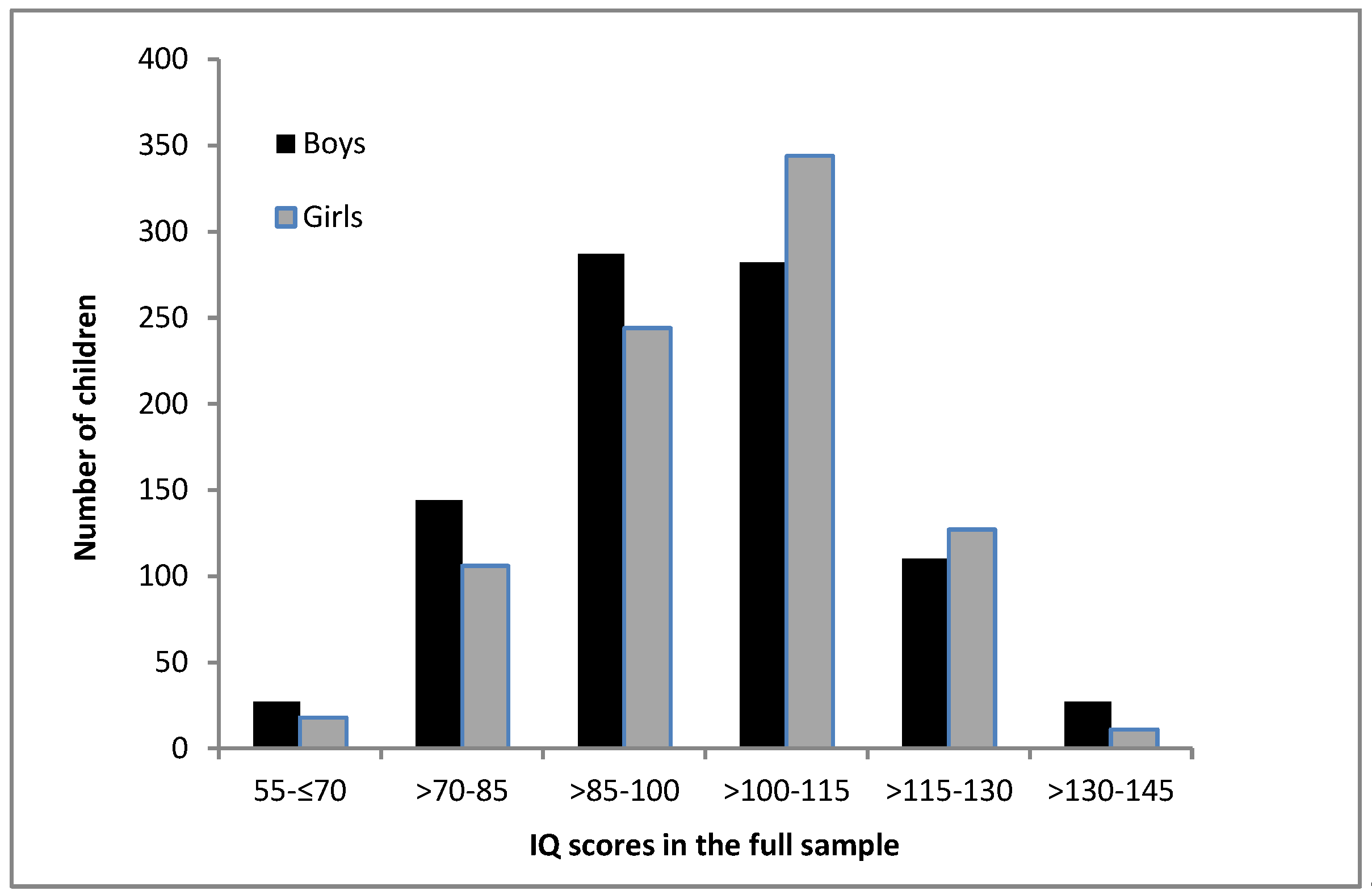
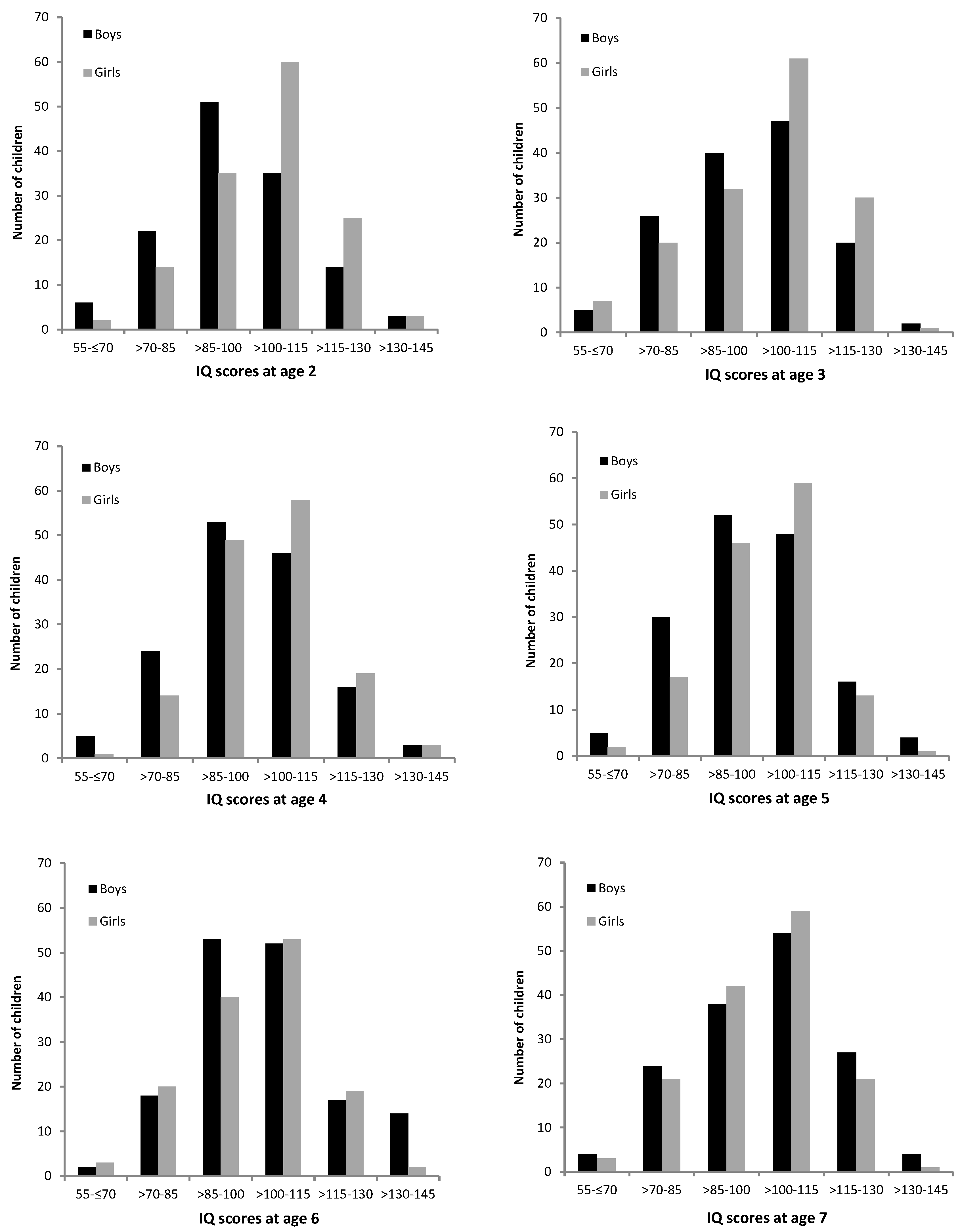
3. Results
4. Discussion
5. Conclusions
6. Limitations and Recommendations for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halpern, D.F. Sex Differences in Cognitive Abilities, 4th ed.; Psychology Press: New York, NY, USA, 2012. [Google Scholar]
- Voyer, D.; Voyer, S.; Bryden, M.P. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychol. Bull. 1995, 117, 250–270. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.S.; Linn, M.C. Gender differences in verbal ability: A meta-analysis. Psychol. Bull. 1988, 104, 53–69. [Google Scholar] [CrossRef]
- Halpern, D.F.; Benbow, C.P.; Geary, D.C.; Gur, R.C.; Shilbey Hyde, J.; Gernsbacher, M.A. The science of sex differences in science and mathematics. Psychol. Sci. Public Interest 2007, 8, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.S. Sex and cognition: Gender and cognitive functions. Curr. Opin. Neurobiol. 2016, 38, 53–56. [Google Scholar] [CrossRef]
- Hyde, J.S. The gender similarities hypothesis. Am. Psychol. 2005, 60, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ardila, A.; Rosselli, M.; Matute, E.; Inozemtseva, O. Gender differences in cognitive development. Dev. Psychol. 2011, 47, 984–990. [Google Scholar] [CrossRef]
- Hyde, J.S. Gender similarities and differences. Annu. Rev. Psychol. 2014, 65, 373–398. [Google Scholar] [CrossRef]
- Miller, D.I.; Halpern, D.F. The new science of cognitive sex differences. Trends Cogn. Sci. 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Palejwala, M.H.; Fine, J.G. Gender differences in latent cognitive abilities in children aged 2 to 7. Intelligence 2015, 48, 96–108. [Google Scholar] [CrossRef]
- Goldbeck, L.; Daseking, M.; Hellwig-Brida, S.; Waldmann, H.C.; Petermann, F. Sex Differences on the German Wechsler Intelligence Test for Children (WISC-IV). J. Individ. Differ. 2010, 31, 22–28. [Google Scholar] [CrossRef]
- Pezzuti, L.; Orsini, A. Are there sex differences in the wechsler intelligence scale for children—Forth edition? Learn. Individ. Differ. 2016, 45, 307–312. [Google Scholar] [CrossRef]
- Aluja-Fabregat, A.; Colom, R.; Abad, F.; Juan-Espinosa, M. Sex differences in general intelligence defined as g among young adolescents. Pers. Individ. Differ. 2000, 28, 813–820. [Google Scholar] [CrossRef]
- Keith, T.Z.; Reynolds, M.R.; Roberts, L.G.; Winter, A.L.; Austin, C.A. Sex differences in latent cognitive abilities ages 5 to 17: Evidence from the differential ability scales—Second edition. Intelligence 2011, 39, 389–404. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Keith, T.Z.; Flanagan, D.P.; Alfonso, V.C. A cross-battery, reference variable, confirmatory factor analytic investigation of the chc taxonomy. J. Sch. Psychol. 2013, 51, 535–555. [Google Scholar] [CrossRef]
- Keith, T.Z.; Reynolds, M.R.; Patel, P.G.; Ridley, K.P. Sex differences in latent cognitive abilities ages 6 to 59: Evidence from the woodcock-johnson iii tests of cognitive abilities. Intelligence 2008, 36, 502–525. [Google Scholar] [CrossRef]
- Harnqvist, K. Gender and grade differences in latent ability variables. Scand. J. Psychol. 1997, 38, 55–62. [Google Scholar] [CrossRef]
- Rosén, M. Gender differences in structure, means and variances of hierarchically ordered ability dimensions. Learn. Instr. 1995, 5, 37–62. [Google Scholar] [CrossRef]
- Calvin, C.M.; Fernandes, C.; Smith, P.; Visscher, P.M.; Deary, I.J. Sex, intelligence and educational achievement in a national cohort of over 175,000 11-year-old schoolchildren in england. Intelligence 2010, 38, 424–432. [Google Scholar] [CrossRef]
- Sellers, A.H.; Burns, W.J.; Guyrke, J. Differences in young children’s iqs on the wechsler preschool and primary scale of intelligence-revised as a function of stratification variables. Appl. Neuropsychol. 2002, 9, 65–73. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence–Revised (WPPSI-R); The Psychological Corporation: San Antonio, TX, USA, 1989. [Google Scholar]
- Burns, C.W.; Reynolds, C.R. Patterns of sex differences in children’s information processing with and without independence from g. J. Sch. Psychol. 1988, 26, 233–242. [Google Scholar] [CrossRef]
- Kaufman, A.S.; Kaufman, N.L. Kaufman Assessment Battery for Children (K-ABC); American Guidance Service: Circle Pines, MN, USA, 1983. [Google Scholar]
- Wechsler, D. Wechsler Primary and Preschool Scale of Intelligence—Fourth Edition (WPPSI-IV); The Psychological Corporation: San Antonio, TX, USA, 2012. [Google Scholar]
- Arden, R.; Plomin, R. Sex differences in variance of intelligence across childhood. Pers. Individ. Differ. 2006, 41, 39–48. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Keith, T.Z.; Ridley, K.P.; Patel, P.G. Sex differences in latent general and broad cognitive abilities for children and youth: Evidence from higher-order mg-macs and mimic models. Intelligence 2008, 36, 236–260. [Google Scholar] [CrossRef]
- Levine, S.C.; Huttenlocher, J.; Taylor, A.; Langrock, A. Early sex differences in spatial skill. Dev. Psychol. 1999, 35, 940–949. [Google Scholar] [CrossRef]
- Frick, A.; Mohring, W. Mental object rotation and motor development in 8- and 10-month-old infants. J. Exp. Child Psychol. 2013, 115, 708–720. [Google Scholar] [CrossRef]
- Quinn, P.C.; Liben, L.S. A sex difference in mental rotation in infants: Convergent evidence. Infancy 2014, 19, 103–116. [Google Scholar] [CrossRef]
- Toivainen, T.; Papageorgiou, K.A.; Tosto, M.G.; Kovas, Y. Sex differences in non-verbal and verbal abilities in childhood and adolescence. Intelligence 2017, 64, 81–88. [Google Scholar] [CrossRef]
- Lynn, R. Sex differences in intelligence and brain size: A developmental theory. Intelligence 1999, 27, 1–12. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Gogtay, N.; Greenstein, D.K.; Wells, E.M.; Wallace, G.L.; Clasen, L.S.; Blumenthal, J.D.; Lerch, J.; Zijdenbos, A.P.; Evans, A.C.; et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007, 36, 1065–1073. [Google Scholar] [CrossRef]
- Penner, A.M.; Paret, M. Gender differences in mathematics achievement: Exploring the early grades and the extremes. Soc. Sci. Res. 2008, 37, 239–253. [Google Scholar] [CrossRef]
- Schwarzer, G.; Freitag, C.; Schum, N. How crawling and manual object exploration are related to the mental rotation abilities of 9-month-old infants. Front. Psychol. 2013, 4, 97. [Google Scholar] [CrossRef]
- Lakin, J.; Gambrell, J. Sex Differences in fluid reasoning: Manifest and latent estimates from the Cognitive Abilities Test. J. Intell. 2014, 2, 36–55. [Google Scholar] [CrossRef]
- Strand, S.; Deary, I.J.; Smith, P. Sex differences in cognitive abilities test scores: A UK national picture. Br. J. Educ. Psychol. 2006, 76, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Carothers, A.; Deary, I.J. Sex differences in variability in general intelligence: A new look at the old question. Perspect. Psychol. Sci. 2008, 3, 518–531. [Google Scholar] [CrossRef]
- Deary, I.J.; Thorpe, G.; Wilson, V.; Starr, J.M.; Whalley, L.J. Population sex differences in IQ at age 11: The Scottish mental survey 1932. Intelligence 2003, 31, 533–542. [Google Scholar] [CrossRef]
- Wierenga, L.M.; Sexton, J.A.; Laake, P.; Giedd, J.N.; Tamnes, C.K. A key characteristic of sex differences in the developing brain: Greater variability in brain structure of boys than girls. Cereb. Cortex 2018, 28, 2741–2751. [Google Scholar] [CrossRef]
- Lehre, A.C.; Lehre, K.P.; Laake, P.; Danbolt, N.C. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev. Psychobiol. 2009, 51, 198–206. [Google Scholar] [CrossRef]
- Tellegen, P.J.; Laros, J.A.; Petermann, F. SON-R 2-8: Non-Verbaler Intelligenztest; Hogrefe: Göttingen, Germany, 2018. [Google Scholar]
- Tellegen, P.J.; Laros, J.A.; Petermann, F. SON-R 2-8: Non-Verbaler Intelligenztest. I. Technisches Manual; Hogrefe: Göttingen, Germany, 2018. [Google Scholar]
- Tellegen, P.J.; Laros, J.A.; Petermann, F. SON-R 2½-7. Non-Verbaler Intelligenztest; Hogrefe: Göttingen, Germany, 2007. [Google Scholar]
- Snijders-Oomen, A.W.M. Intelligentieonderzoek van Doofstomme Kinderen: Een Nieuwe Testschaal. Proefschrift; Berkhout: Nijmegen, The Netherlands, 1943. [Google Scholar]
- Snijders, J.T.; Tellegen, P.J.; Laros, J.A. Snijders-Oomen Nonverbaler Intelligenztest SON-R 5.5-17; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Schneider, W.J.; McGrew, K.S. The Cattell-Horn-Carroll Theory of Cognitive Abilities, in Contemporary Intellectual Assessment: Theories, Tests and Issues; Flanagan, D.P., Harrison, P.L., Eds.; Guilford Press: New York, NY, USA, 2018. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, MI, USA, 1988. [Google Scholar]
- Feingold, A. Gender differences in intellectual abilities—A cross-cultural-perspective. Sex Roles 1994, 30, 81–92. [Google Scholar] [CrossRef]
- Maccoby, E.E.; Jacklin, C.N. The Psychology of Sex Differences; Stanford University Press: Stanford, CA, USA, 1974. [Google Scholar]
- Hedges, L.V.; Nowell, A. Sex-differences in mental test-scores, variability, and numbers of high-scoring individuals. Science 1995, 269, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Schaie, K.W.; Willis, S.L.; Pennak, S. An historical framework for cohort differences in intelligence. Res. Hum. Dev. 2005, 2, 43–67. [Google Scholar] [CrossRef]
- Walter, F.; Petermann, F.; Daseking, M. Vorhersage von kognitiven Fähigkeiten in der WPSSI-IV durch den ET 6-6-R [The prediction of cognitive ability in the WPPSI-IV by the ET 6-6-R]. Kindh. Entwickl. 2018, 3, 131–141. [Google Scholar] [CrossRef]
- Jaščenoka, J.; Walter, F.; Petermann, F.; Korsch, F.; Fiedler, S. Zum Zusammenhang von motorischer und kognitiver´Entwicklung im Vorschulalter [The relationship between motor and cognitive development in preschool age]. Kindh. Entwickl. 2018, 3, 142–152. [Google Scholar] [CrossRef]
| Dutch | German | Full Sample | Sex | Age | ||
|---|---|---|---|---|---|---|
| Age Group | N | N | N | Boys | Girls | Mean (SD) |
| 2 years | 144 | 126 | 270 | 131 | 139 | 2.52 (0.36) |
| 3 years | 165 | 126 | 291 | 140 | 151 | 3.50 (0.28) |
| 4 years | 165 | 126 | 291 | 147 | 144 | 4.50 (0.29) |
| 5 years | 165 | 128 | 293 | 155 | 138 | 5.52 (0.29) |
| 6 years | 162 | 128 | 290 | 153 | 137 | 6.48 (0.30) |
| 7 years | 164 | 128 | 292 | 151 | 141 | 7.48 (0.27) |
| Overall | 965 | 762 | 1727 | 877 | 850 | 5.03 (1.71) |
| Boys | Girls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Variable | M | SD | M | SD | Δ | F | p | p corr. | d | VR |
| 2 years | Puzzles | 9.82 | 2.86 | 10.09 | 2.69 | −0.27 | 0.64 | 0.426 | 2.556 | −0.10 | 1.07 |
| Patterns | 10.08 | 3.44 | 11.53 | 3.26 | −1.45 | 12.64 | 0.000 | 0.000 | −0.43 | 1.06 | |
| Mosaics | 9.70 | 3.31 | 10.35 | 2.94 | −0.64 | 2.85 | 0.093 | 0.558 | −0.21 | 1.13 | |
| Categories | 9.79 | 3.18 | 10.51 | 2.80 | −0.72 | 3.87 | 0.050 | 0.300 | −0.24 | 1.14 | |
| Situations | 8.79 | 3.55 | 10.09 | 3.49 | −1.30 | 9.19 | 0.003 | 0.018 | −0.37 | 1.02 | |
| Analogies | 9.61 | 3.39 | 10.43 | 3.41 | −0.82 | 3.92 | 0.049 | 0.294 | −0.24 | 0.99 | |
| 3 years | Puzzles | 10.02 | 3.17 | 10.05 | 3.01 | −0.02 | 0.00 | 0.945 | 5.670 | −0.01 | 1.05 |
| Patterns | 10.44 | 3.16 | 10.96 | 3.15 | −0.52 | 1.95 | 0.163 | 0.978 | −0.17 | 1.00 | |
| Mosaics | 9.81 | 3.50 | 10.07 | 3.25 | −0.25 | 0.41 | 0.525 | 3.150 | −0.08 | 1.08 | |
| Categories | 9.74 | 3.09 | 10.06 | 2.92 | −0.32 | 0.81 | 0.370 | 2.220 | −0.11 | 1.06 | |
| Situations | 9.43 | 2.86 | 10.01 | 2.96 | −0.58 | 2.87 | 0.092 | 0.552 | −0.20 | 0.97 | |
| Analogies | 10.14 | 3.00 | 10.09 | 2.94 | 0.04 | 0.02 | 0.902 | 5.412 | −0.02 | 1.02 | |
| 4 years | Puzzles | 9.99 | 2.87 | 10.09 | 2.90 | −0.10 | 0.08 | 0.774 | 4.644 | −0.04 | 0.99 |
| Patterns | 10.05 | 2.95 | 10.42 | 2.08 | −0.37 | 1.52 | 0.219 | 1.314 | −0.15 | 1.42 | |
| Mosaics | 9.90 | 2.90 | 10.54 | 2.39 | −0.64 | 4.17 | 0.042 | 0.252 | −0.24 | 1.22 | |
| Categories | 9.53 | 3.18 | 10.62 | 3.05 | −1.09 | 8.86 | 0.003 | 0.018 | −0.35 | 1.04 | |
| Situations | 9.59 | 2.86 | 10.05 | 2.68 | −0.46 | 1.97 | 0.161 | 0.966 | −0.17 | 1.07 | |
| Analogies | 9.65 | 2.82 | 10.16 | 2.80 | −0.51 | 2.37 | 0.125 | 0.750 | −0.18 | 1.01 | |
| 5 years | Puzzles | 9.91 | 3.01 | 10.09 | 3.00 | −0.18 | 0.28 | 0.600 | 3.600 | −0.06 | 1.00 |
| Patterns | 9.46 | 3.07 | 10.02 | 2.63 | −0.56 | 2.82 | 0.094 | 0.564 | −0.20 | 1.17 | |
| Mosaics | 10.08 | 3.44 | 9.72 | 2.53 | 0.36 | 1.02 | 0.313 | 1.878 | 0.12 | 1.36 | |
| Categories | 9.72 | 3.16 | 10.09 | 2.90 | −0.37 | 1.09 | 0.298 | 1.788 | −0.12 | 1.09 | |
| Situations | 9.87 | 3.10 | 10.37 | 2.66 | −0.50 | 2.16 | 0.143 | 0.858 | −0.17 | 1.17 | |
| Analogies | 9.72 | 3.13 | 10.20 | 2.88 | −0.47 | 1.80 | 0.181 | 1.086 | −0.16 | 1.08 | |
| 6 years | Puzzles | 10.07 | 3.34 | 10.09 | 3.13 | −0.02 | 0.00 | 0.952 | 5.712 | −0.01 | 1.07 |
| Patterns | 10.29 | 3.35 | 10.30 | 3.16 | −0.01 | 0.00 | 0.976 | 5.856 | −0.01 | 1.06 | |
| Mosaics | 10.50 | 3.15 | 9.58 | 2.79 | 0.92 | 6.86 | 0.009 | 0.054 | 0.31 | 1.13 | |
| Categories | 10.23 | 3.42 | 10.19 | 2.84 | 0.04 | 0.01 | 0.916 | 5.496 | 0.01 | 1.20 | |
| Situations | 10.50 | 3.05 | 10.31 | 3.06 | 0.19 | 0.28 | 0.599 | 3.594 | 0.06 | 1.00 | |
| Analogies | 10.46 | 3.21 | 10.15 | 3.18 | 0.30 | 0.66 | 0.419 | 2.514 | 0.10 | 1.01 | |
| 7 years | Puzzles | 10.07 | 3.24 | 10.00 | 3.21 | 0.07 | 0.04 | 0.847 | 5.082 | 0.02 | 1.01 |
| Patterns | 10.15 | 3.25 | 10.27 | 3.03 | −0.12 | 0.11 | 0.737 | 4.422 | −0.04 | 1.07 | |
| Mosaics | 10.55 | 3.20 | 9.52 | 2.91 | 1.03 | 8.16 | 0.005 | 0.030 | 0.34 | 1.10 | |
| Categories | 9.75 | 3.09 | 10.10 | 2.84 | −0.34 | 0.98 | 0.324 | 1.944 | −0.12 | 1.09 | |
| Situations | 10.62 | 3.07 | 10.78 | 2.92 | −0.16 | 0.22 | 0.641 | 3.846 | −0.05 | 1.05 | |
| Analogies | 9.87 | 2.88 | 9.88 | 2.65 | −0.01 | 0.00 | 0.987 | 5.922 | −0.01 | 1.09 | |
| Boys | Girls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Variable | M | SD | M | SD | Δ | F | p | p corr. | d | VR |
| 2 years | IQ PS | 99.09 | 15.50 | 104.06 | 13.89 | −4.97 | 7.70 | 0.006 | 0.018 | −0.34 | 1.12 |
| IQ RS | 96.32 | 16.19 | 102.09 | 15.99 | −5.77 | 8.68 | 0.003 | 0.009 | −0.36 | 1.01 | |
| IQ | 97.44 | 15.55 | 103.37 | 14.20 | −5.93 | 10.73 | 0.001 | 0.003 | −0.40 | 1.10 | |
| 3 years | IQ PS | 100.51 | 16.39 | 102.15 | 16.11 | −1.64 | 0.74 | 0.391 | 1.173 | −0.10 | 1.02 |
| IQ RS | 98.24 | 15.84 | 100.44 | 15.18 | −2.19 | 1.46 | 0.229 | 0.687 | −0.14 | 1.04 | |
| IQ | 99.57 | 16.07 | 101.52 | 15.59 | −1.95 | 1.11 | 0.294 | 0.882 | −0.12 | 1.03 | |
| 4 years | IQ PS | 100.01 | 14.95 | 102.02 | 12.25 | −2.01 | 1.57 | 0.212 | 0.636 | −0.15 | 1.22 |
| IQ RS | 97.22 | 16.34 | 101.89 | 14.48 | −4.67 | 6.65 | 0.010 | 0.030 | −0.30 | 1.13 | |
| IQ | 98.50 | 15.25 | 102.17 | 13.01 | −3.67 | 4.87 | 0.028 | 0.084 | −0.26 | 1.17 | |
| 5 years | IQ PS | 98.99 | 15.54 | 99.58 | 13.16 | −0.59 | 0.12 | 0.729 | 2.187 | −0.04 | 1.18 |
| IQ RS | 98.19 | 16.49 | 101.35 | 13.60 | −3.16 | 3.16 | 0.077 | 0.231 | −0.21 | 1.21 | |
| IQ | 98.46 | 15.75 | 100.38 | 12.91 | −1.92 | 1.28 | 0.258 | 0.774 | −0.13 | 1.22 | |
| 6 years | IQ PS | 101.76 | 16.17 | 100.18 | 15.07 | 1.58 | 0.74 | 0.391 | 1.173 | 0.10 | 1.07 |
| IQ RS | 102.76 | 16.93 | 101.66 | 15.04 | 1.10 | 0.34 | 0.561 | 1.683 | 0.07 | 1.13 | |
| IQ | 102.41 | 16.05 | 100.79 | 14.59 | 1.62 | 0.80 | 0.372 | 1.116 | 0.11 | 1.10 | |
| 7 years | IQ PS | 101.41 | 16.09 | 99.44 | 15.08 | 1.97 | 1.16 | 0.282 | 0.846 | 0.13 | 1.07 |
| IQ RS | 100.55 | 16.00 | 101.65 | 14.65 | −1.10 | 0.38 | 0.540 | 1.620 | −0.07 | 1.09 | |
| IQ | 101.16 | 16.03 | 100.65 | 14.60 | 0.51 | 0.08 | 0.778 | 2.334 | 0.03 | 1.10 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buczyłowska, D.; Ronniger, P.; Melzer, J.; Petermann, F. Sex Similarities and Differences in Intelligence in Children Aged Two to Eight: Analysis of SON-R 2–8 Scores. J. Intell. 2019, 7, 11. https://0-doi-org.brum.beds.ac.uk/10.3390/jintelligence7020011
Buczyłowska D, Ronniger P, Melzer J, Petermann F. Sex Similarities and Differences in Intelligence in Children Aged Two to Eight: Analysis of SON-R 2–8 Scores. Journal of Intelligence. 2019; 7(2):11. https://0-doi-org.brum.beds.ac.uk/10.3390/jintelligence7020011
Chicago/Turabian StyleBuczyłowska, Dorota, Pola Ronniger, Jessica Melzer, and Franz Petermann. 2019. "Sex Similarities and Differences in Intelligence in Children Aged Two to Eight: Analysis of SON-R 2–8 Scores" Journal of Intelligence 7, no. 2: 11. https://0-doi-org.brum.beds.ac.uk/10.3390/jintelligence7020011





