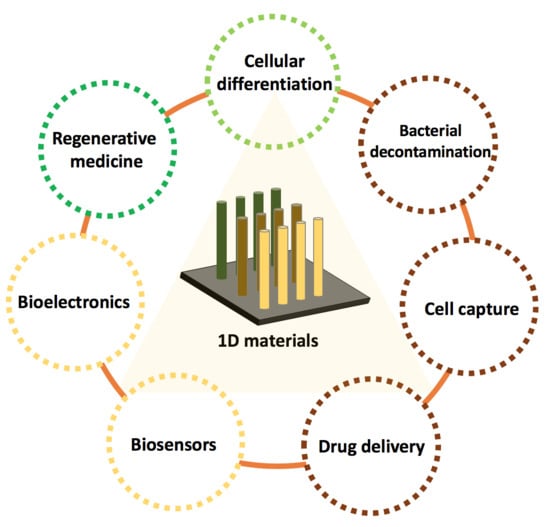
On the Interaction between 1D Materials and Living Cells
Abstract
:1. Introduction
2. Cells at the Interface with 1D Materials
3. Silicon-Based 1D Nanomaterials
Novel Applications in Drug Delivery and Cell Fate Regulation
4. TiO2-Based 1D Materials
4.1. Applications in Bone Regeneration
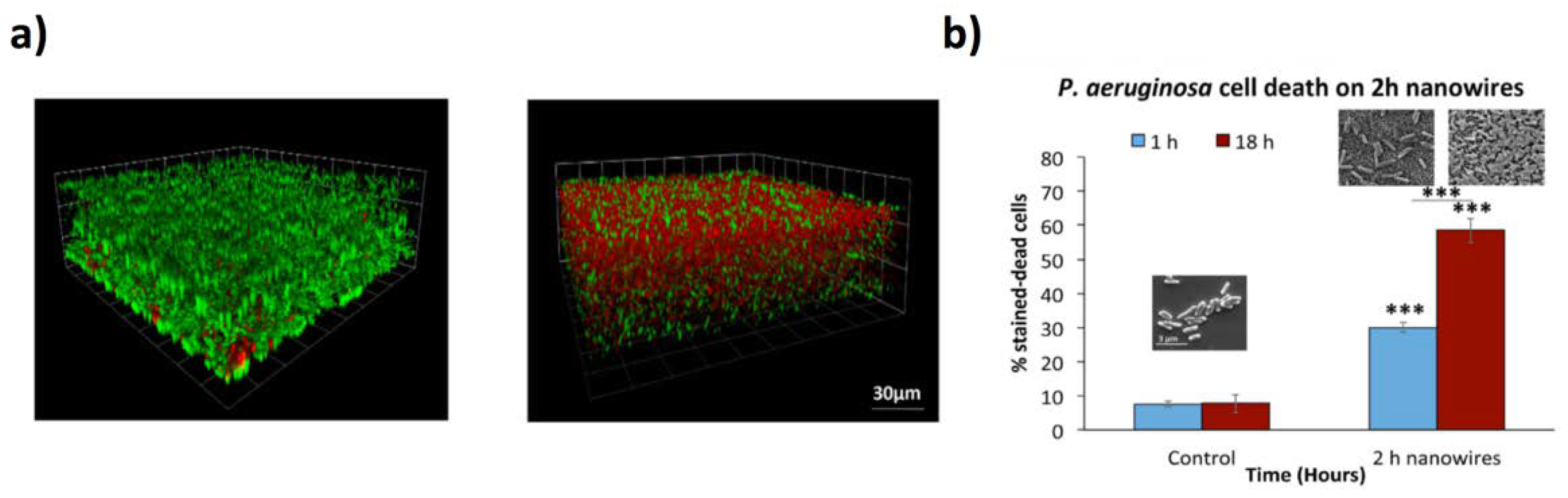
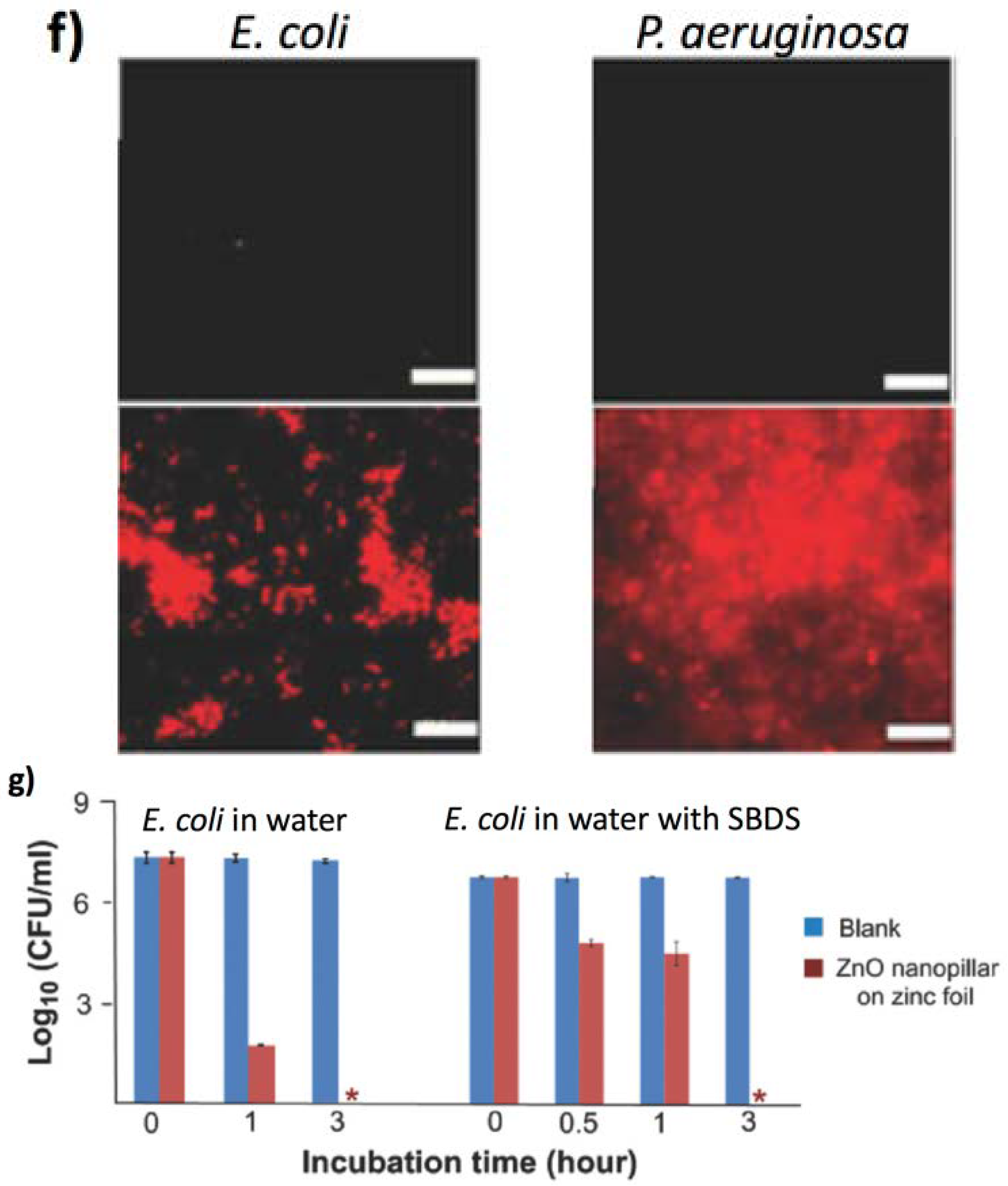
4.2. Tools for Bacterial Decontamination
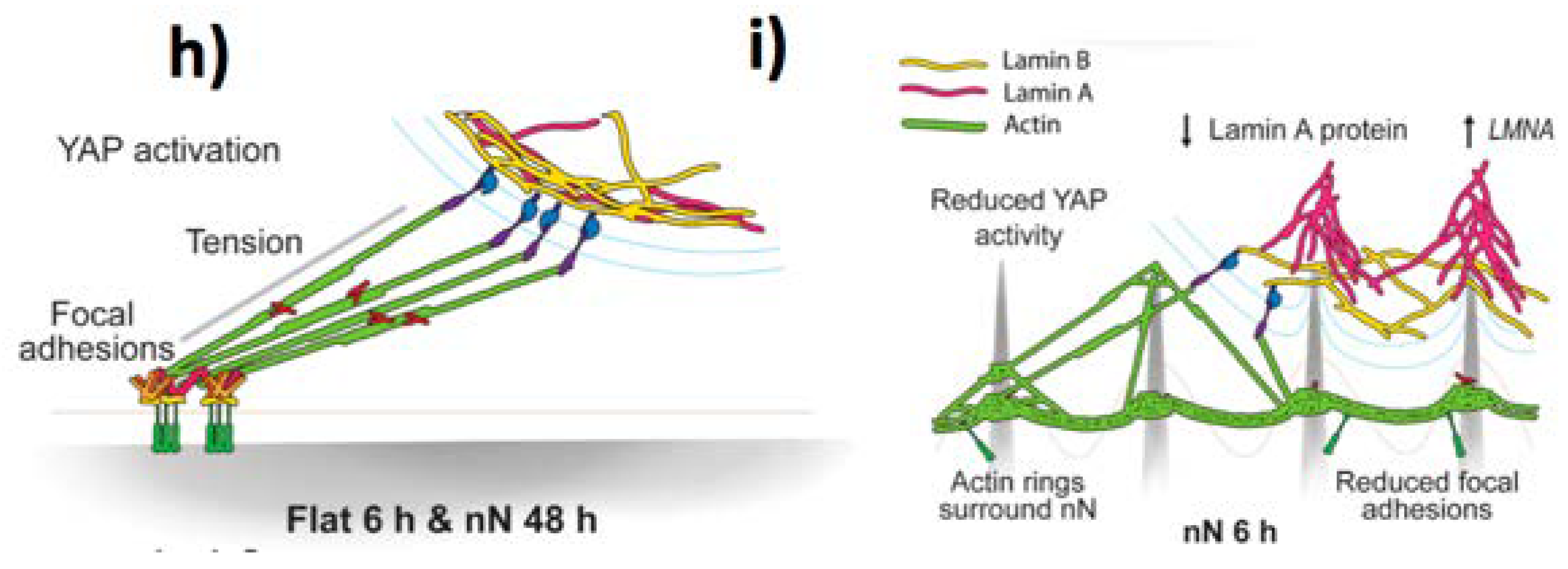
4.2.1. Biomechanics Effects
4.2.2. Photocatalysis
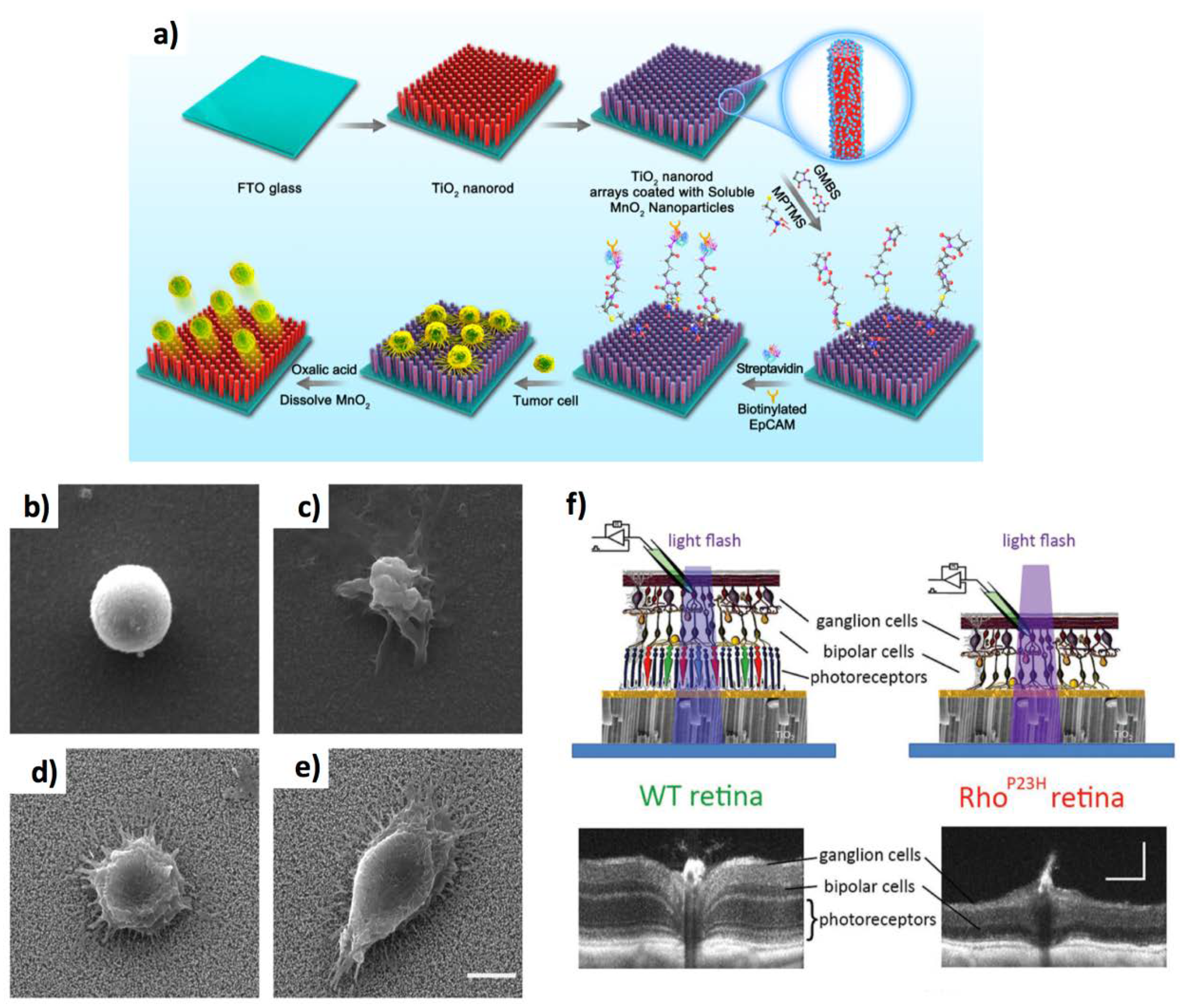
4.3. Targeted Cancer Capture, Targeted Therapy, and Artificial Retinas Devices
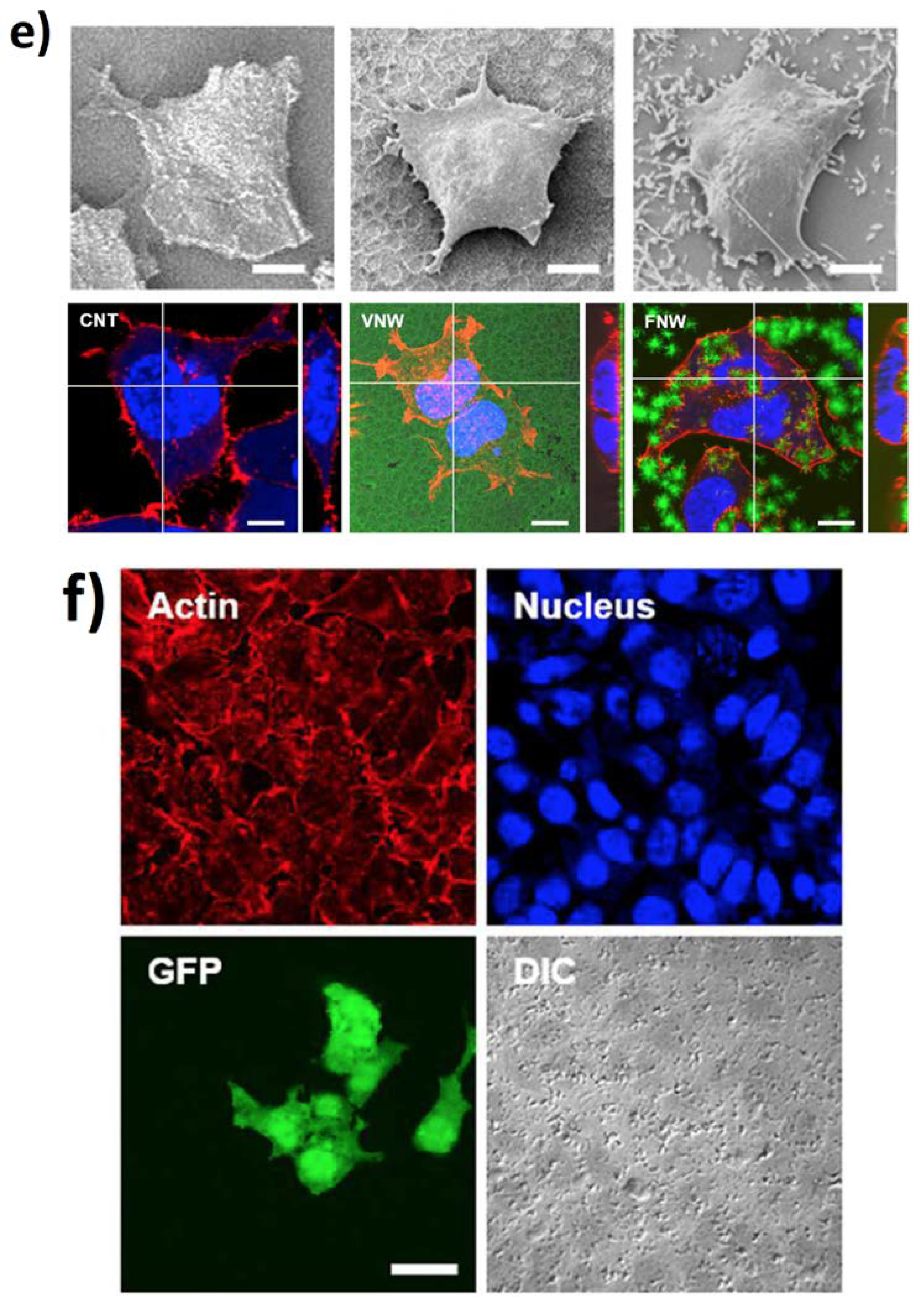
5. Zinc Oxide-Based 1D Materials
5.1. Toxicity Issues: A Cell-Dependent Relation?
5.2. New Frontiers in Regenerative Medicine, Drug Delivery, and Neuromorphic Devices
6. Carbon Nanotubes
6.1. CNTs-Powered Soft Robotics
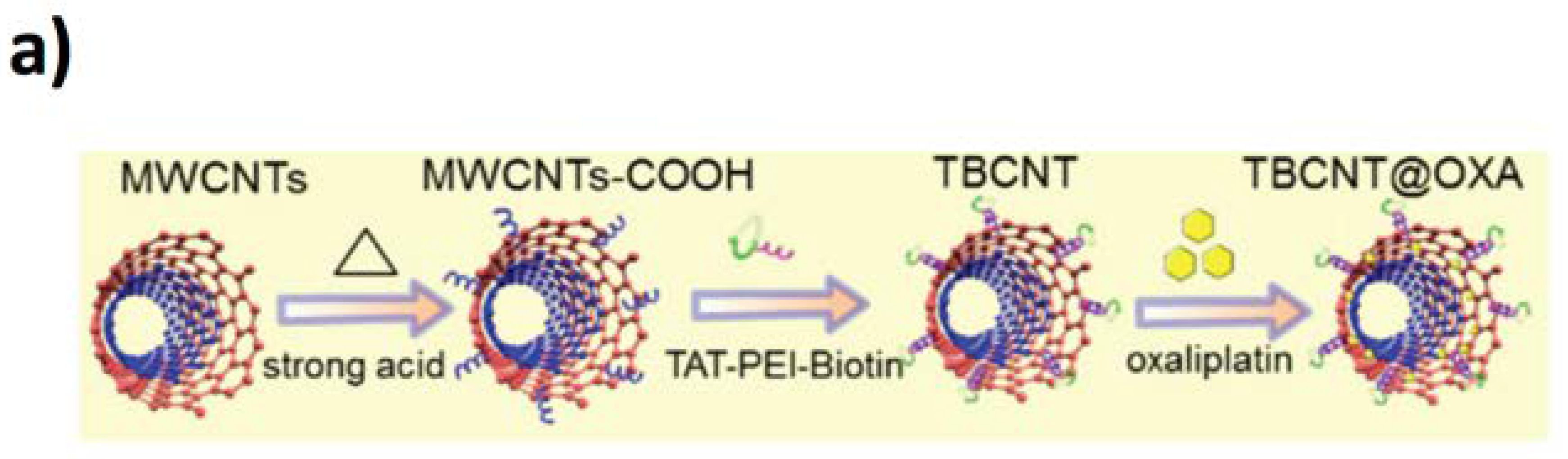
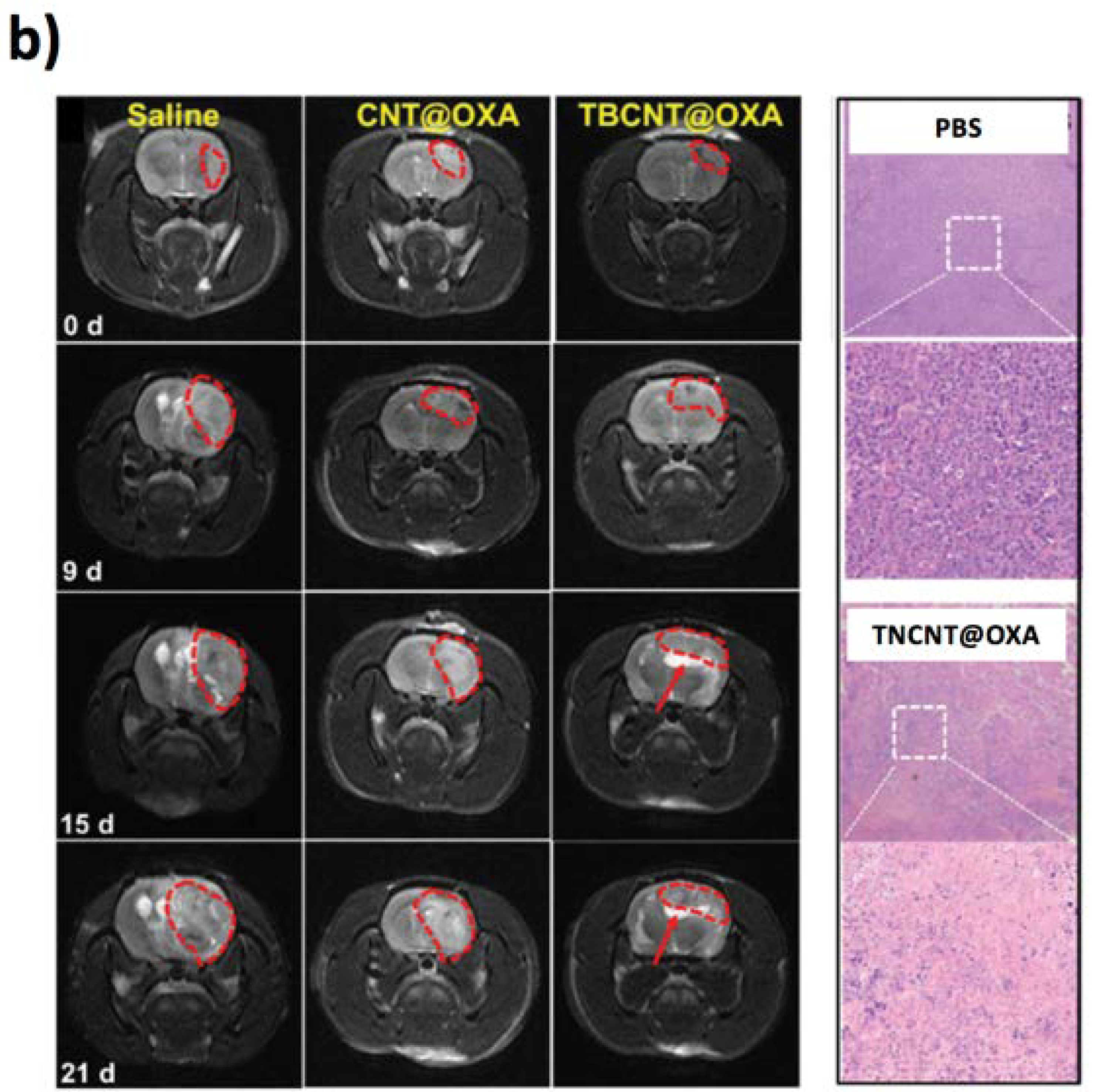
6.2. CNTs-Based Tools for Cellular Stimulation, Cancer Therapy, and Drug Delivery
7. 1D Polymeric Materials
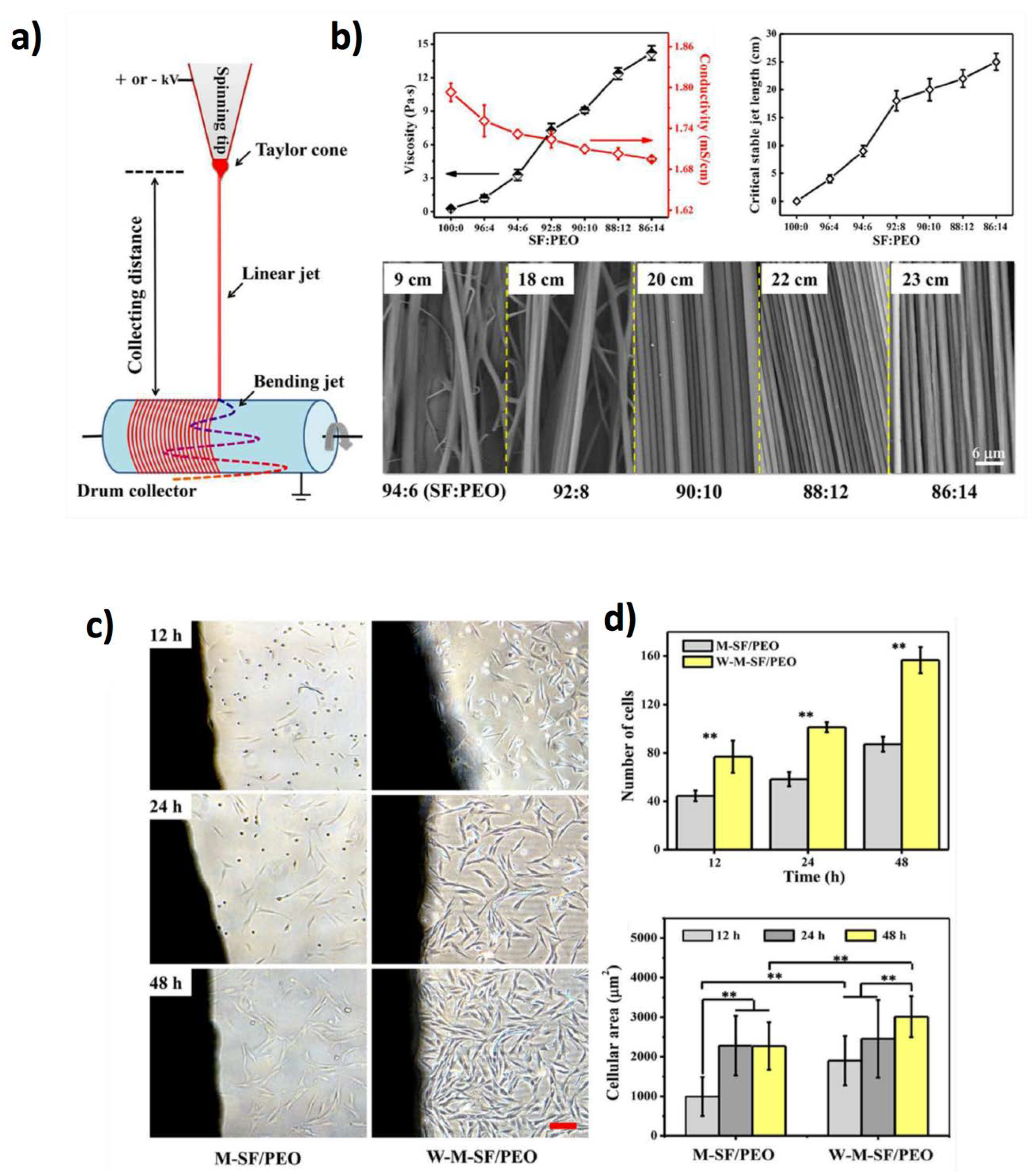
7.1. Electrospun Polymers: From Natural to Synthetic Polymers
7.2. 1D Polymeric Biointerfaces for Cellular Electric Stimulation
7.3. Cancer Cells Capture, Bactericides Agents and Drug Delivery
7.4. Antibacterial 1D Polymeric Biointerfaces
8. DNA and Peptide 1D Materials
8.1. 1D DNA Materials
8.2. 1D Peptide Materials
9. Conclusions, Challenges and Opportunities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arrabito, G.; Reisewitz, S.; Dehmelt, L.; Bastiaens, P.I.; Pignataro, B.; Schroeder, H.; Niemeyer, C.M. Biochips for Cell Biology by Combined Dip-Pen Nanolithography and DNA-Directed Protein Immobilization. Small 2013, 9, 4243–4249. [Google Scholar] [CrossRef] [PubMed]
- Gandor, S.; Reisewitz, S.; Venkatachalapathy, M.; Arrabito, G.; Reibner, M.; Schröder, H.; Ruf, K.; Niemeyer, C.M.; Bastiaens, P.I.H.; Dehmelt, L. A protein-interaction array inside a living cell. Angew. Chem. Int. Ed. 2013, 52, 4790–4794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrabito, G.; Schroeder, H.; Schröder, K.; Filips, C.; Marggraf, U.; Dopp, C.; Venkatachalapathy, M.; Dehmelt, L.; Bastiaens, P.I.H.; Neyer, A.; et al. Configurable low-cost plotter device for fabrication of multi-color sub-cellular scale microarrays. Small 2014, 10, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.D.; Higgins, M.J.; Moulton, S.E.; Wallace, G.G. Nano-bioelectronics via dip-pen nanolithography. J. Mater. Chem. C 2015, 3, 6431–6444. [Google Scholar] [CrossRef] [Green Version]
- McGuire, A.F.; Santoro, F.; Cui, B. Interfacing Cells with Vertical Nanoscale Devices: Applications and Characterization. Annu. Rev. Anal. Chem. 2018, 11, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, B.; Zou, Y.; Zhu, D.; Lei, L.; Zhao, D.; Nie, H. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional biomaterials for cell fate regulation. Adv. Drug Deliv. Rev. 2018, 132, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, M.Y.; Tian, B. Talking to Cells: Semiconductor Nanomaterials at the Cellular Interface. Adv. Biosyst. 2018, 2, 1700242. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A complementary definition of nanomaterial. Nano Today 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, L.A.; Dalby, M.J. Nanotopography-potential relevance in the stem cell niche. Biomater. Sci. 2014, 2, 1574–1594. [Google Scholar] [CrossRef] [PubMed]
- Cantini, M.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The Plot Thickens: The Emerging Role of Matrix Viscosity in Cell Mechanotransduction. Adv. Healthc. Mater. 2020, 9, 1901259. [Google Scholar] [CrossRef] [PubMed]
- Kassianidou, E.; Probst, D.; Jäger, J.; Lee, S.; Roguet, A.L.; Schwarz, U.S.; Kumar, S. Extracellular Matrix Geometry and Initial Adhesive Position Determine Stress Fiber Network Organization during Cell Spreading. Cell Rep. 2019, 27, 1897–1909.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estabridis, H.M.; Jana, A.; Nain, A.; Odde, D.J. Cell Migration in 1D and 2D Nanofiber Microenvironments. Ann. Biomed. Eng. 2018, 46, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goor, O.J.G.M.; Hendrikse, S.I.S.; Dankers, P.Y.W.; Meijer, E.W. From supramolecular polymers to multi-component biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrabito, G.; Errico, V.; Zhang, Z.; Han, W.; Falconi, C. Nanotransducers on printed circuit boards by rational design of high-density, long, thin and untapered ZnO nanowires. Nano Energy 2018, 46, 54–62. [Google Scholar] [CrossRef]
- Liu, R.; Chen, R.; Elthakeb, A.T.; Lee, S.H.; Hinckley, S.; Khraiche, M.L.; Scott, J.; Pre, D.; Hwang, Y.; Tanaka, A.; et al. High Density Individually Addressable Nanowire Arrays Record Intracellular Activity from Primary Rodent and Human Stem Cell Derived Neurons. Nano Lett. 2017, 17, 2757–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, F.; Zhao, W.; Joubert, L.M.; Duan, L.; Schnitker, J.; Van De Burgt, Y.; Lou, H.Y.; Liu, B.; Salleo, A.; Cui, L.; et al. Revealing the Cell-Material Interface with Nanometer Resolution by Focused Ion Beam/Scanning Electron Microscopy. ACS Nano 2017, 11, 8320–8328. [Google Scholar] [CrossRef] [PubMed]
- Dipalo, M.; Caprettini, V.; Bruno, G.; Caliendo, F.; Garma, L.D.; Melle, G.; Dukhinova, M.; Siciliano, V.; Santoro, F.; De Angelis, F. Membrane Poration Mechanisms at the Cell–Nanostructure Interface. Adv. Biosyst. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Kong, Y.; Ma, B.; Liu, F.; Chen, D.; Zhang, S.; Duan, J.; Huang, Y.; Sang, Y.; Wang, J.; Li, D.; et al. Cellular Stemness Maintenance of Human Adipose-Derived Stem Cells on ZnO Nanorod Arrays. Small 2019, 15, 1904099. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-M.; Song, W.-J.; Bi, H.; Wang, J.; Wu, W.-L.; Sun, J.; Yu, M. Cytotoxicity and cellular uptake of iron nanowires. Biomaterials 2010, 31, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Digabel, J.L.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Nina, B.; Sara, B.; Jessica, B.; Trine, B.; Jesper, N.; Martinez, K.L. Towards a Better Prediction of Cell Settling on Nanostructure Arrays—Simple Means to Complicated Ends. Adv. Funct. Mater. 2015, 25, 3246–3255. [Google Scholar]
- Xie, X.; Xu, A.M.; Angle, M.R.; Tayebi, N.; Verma, P.; Melosh, N.A. Mechanical Model of Vertical Nanowire Cell Penetration. Nano Lett. 2013, 13, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, Y.; Dang, Z.; Li, J.; Li, X.; Yang, Y.; Chen, T. Origin of efficiency enhancement in cell capture on nanostructured arrays. J. Mater. Sci. 2019, 54, 4236–4245. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Sun, J.; Dang, Z.; Li, J.; Li, X.; Chen, T. The effects of surface topography of nanostructure arrays on cell adhesion. Phys. Chem. Chem. Phys. 2018, 20, 22946–22951. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, W. Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Zeitschrift Naturforsch. C 2014, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T. Enhancement and suppression effects of a nanopatterned surface on bacterial adhesion. Phys. Rev. E 2016, 93, 52419. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Dasgupta, S.; Schnitker, J.; Auth, T.; Neumann, E.; Panaitov, G.; Gompper, G.; Offenhäusser, A. Interfacing Electrogenic Cells with 3D Nanoelectrodes: Position, Shape, and Size Matter. ACS Nano 2014, 8, 6713–6723. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.Y.; Zhao, W.; Zeng, Y.; Cui, B. The Role of Membrane Curvature in Nanoscale Topography-Induced Intracellular Signaling. Acc. Chem. Res. 2018, 51, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, C. Nanoneedle-Based Sensing in Biological Systems. ACS Sens. 2017, 2, 1086–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Dai, X.; Lieber, C.M. Advances in nanowire bioelectronics. Rep. Prog. Phys. 2017, 80, 16701. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; Tian, B. Rational Design of Semiconductor Nanostructures for Functional Subcellular Interfaces. Acc. Chem. Res. 2018, 51, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Acaron Ledesma, H.; Tian, B. Nanoscale silicon for subcellular biointerfaces. J. Mater. Chem. B 2017, 5, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, M.Y.; Elbaz, B.; Nair, V.; Schaumann, E.N.; Yamamoto, N.; Sarma, N.; Matino, L.; Santoro, F.; Tian, B. Silicon Nanowires for Intracellular Optical Interrogation with Subcellular Resolution. Nano Lett. 2020, 20, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Timko, B.P.; Yu, G.; Fang, Y.; Greytak, A.B.; Zheng, G.; Lieber, C.M. Detection, Stimulation, and Inhibition of Neuronal Signals with High-Density Nanowire Transistor Arrays. Science 2006, 313, 1100–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, R.G.; Petkov, N.; Holmes, J.D. Semiconductor nanowire fabrication by bottom-up and top-down paradigms. Chem. Mater. 2012, 24, 1975–1991. [Google Scholar] [CrossRef] [Green Version]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Tang, Y.H.; Wang, N.; Yu, D.P.; Lee, C.S.; Bello, I.; Lee, S.T. Silicon nanowires prepared by laser ablation at high temperature. Appl. Phys. Lett. 1998, 72, 1835–1837. [Google Scholar] [CrossRef]
- Alfredo, M.; Morales, C.M.L. A Laser Ablation Method for the Synthesis of. Science 1998, 279, 208–211. [Google Scholar]
- Xu, A.M.; Aalipour, A.; Leal-Ortiz, S.; Mekhdjian, A.H.; Xie, X.; Dunn, A.R.; Garner, C.C.; Melosh, N.A. Quantification of nanowire penetration into living cells. Nat. Commun. 2014, 5, 3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, J.F.; Parameswaran, R.; Murray, G.; Wang, Y.; Burke, M.; Tian, B. Cellular uptake and dynamics of unlabeled freestanding silicon nanowires. Sci. Adv. 2016, 2, 1601039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andolfi, L.; Murello, A.; Cassese, D.; Ban, J.; Dal Zilio, S.; Lazzarino, M. High aspect ratio silicon nanowires control fibroblast adhesion and cytoskeleton organization. Nanotechnology 2017, 28, 155102. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, C.; De Rosa, E.; Martinez, J.O.; Liu, X.; Steele, J.; Stevens, M.M.; Tasciotti, E. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat. Mater. 2015, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, C.; Campagnolo, P.; Almeida, C.S.; Abbassi-Ghadi, N.; Chow, L.W.; Hanna, G.B.; Stevens, M.M. Mapping Local Cytosolic Enzymatic Activity in Human Esophageal Mucosa with Porous Silicon Nanoneedles. Adv. Mater. 2015, 27, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Chiappini, C.; Penders, J.; Leonardo, V.; Seong, H.; Rothery, S.; Korchev, Y.; Shevchuk, A.; Stevens, M.M. Porous Silicon Nanoneedles Modulate Endocytosis to Deliver Biological Payloads. Adv. Mater. 2019, 31, 1806788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, S.-W.; Lin, H.-I.; Hui-Chun Ho, J.; Shih, Y.-R.V.; Chen, H.-F.; Yen, T.-J.; Lee, O.K. Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires. Biomaterials 2012, 33, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, C.R.; Arl, D.; Grysan, P.; Khan, I.; Durrieu, S.; Krishnamoorthy, S.; Durrieu, M.-C. Controlled Nanoscale Topographies for Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2019, 11, 8858–8866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Q.; Fang, L.; Wei, J.; Xiao, G.; Lv, M.; Ma, Q.; Liu, C.; Wang, W. Silicon nanowires enhanced proliferation and neuronal differentiation of neural stem cell with vertically surface microenvironment. J. Biomater. Sci. Polym. Ed. 2017, 28, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, S.; Kim, D.; Kang, D.H.; Yang, K.; Cho, S.W.; Lee, J.S.; Choi, I.S.; Kang, K.; Yoon, M.H. Strong contact coupling of neuronal growth cones with height-controlled vertical silicon nanocolumns. Nano Res. 2018, 11, 2532–2543. [Google Scholar] [CrossRef]
- Hansel, C.S.; Crowder, S.W.; Cooper, S.; Gopal, S.; João Pardelha da Cruz, M.; de Oliveira Martins, L.; Keller, D.; Rothery, S.; Becce, M.; Cass, A.E.G.; et al. Nanoneedle-Mediated Stimulation of Cell Mechanotransduction Machinery. ACS Nano 2019, 13, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Ouyang, H.; Peng, M.; Jin, Y.; Zhang, Y.; Liu, Z.; Zou, Y.; Zhao, C.; Fan, Y.; Zhai, J.; et al. Assessment of extracellular matrix modulation of cell traction force by using silicon nanowire array. Nano Energy 2018, 50, 504–512. [Google Scholar] [CrossRef]
- Cui, H.; Wang, B.; Wang, W.; Hao, Y.; Liu, C.; Song, K.; Zhang, S.; Wang, S. Frosted Slides Decorated with Silica Nanowires for Detecting Circulating Tumor Cells from Prostate Cancer Patients. ACS Appl. Mater. Interfaces 2018, 10, 19545–19553. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, K.; Liu, J.; Yu, Z.T.F.; Xu, X.; Zhao, L.; Lee, T.; Lee, E.K.; Reiss, J.; Lee, Y.K.; et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed. 2011, 50, 3084–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, H.; Jiao, J.; Chen, K.J.; Owens, G.E.; Kamei, K.I.; Sun, J.; Sherman, D.J.; Behrenbruch, C.P.; Wu, H.; et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. Int. Ed. 2009, 48, 8970–8973. [Google Scholar] [CrossRef] [PubMed]
- Park, G.S.; Kwon, H.; Kwak, D.W.; Park, S.Y.; Kim, M.; Lee, J.H.; Han, H.; Heo, S.; Li, X.S.; Lee, J.H.; et al. Full surface embedding of gold clusters on silicon nanowires for efficient capture and photothermal therapy of circulating tumor cells. Nano Lett. 2012, 12, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, Y.; Liu, Y. Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors. Nanoscale 2014, 6, 12482–12489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, S.; Zhao, H.; Zhao, L.; Shen, Q.; Wei, K.S.; Suh, D.Y.; Nakao, A.; Garcia, M.A.; Song, M.; Lee, T.; et al. Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures. Adv. Mater. 2013, 25, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, X.; Meng, J.; Zhang, P.; Yang, G.; Su, B.; Sun, K.; Chen, L.; Han, D.; Wang, S.; et al. Hydrophobic interaction-mediated capture and release of cancer cells on thermoresponsive nanostructured surfaces. Adv. Mater. 2013, 25, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jiang, Y.; Acaron Ledesma, H.; Yi, J.; Gao, X.; Weiss, D.E.; Shi, F.; Tian, B. Texturing Silicon Nanowires for Highly Localized Optical Modulation of Cellular Dynamics. Nano Lett. 2018, 18, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Miccichè, C.; Arrabito, G.; Amato, F.; Buscarino, G.; Agnello, S.; Pignataro, B. Inkjet printing Ag nanoparticles for SERS hot spots. Anal. Methods 2018, 10, 3215–3223. [Google Scholar] [CrossRef]
- Arrabito, G.; Cavaleri, F.; Montalbano, V.; Vetri, V.; Leone, M.; Pignataro, B. Monitoring few molecular binding events in scalable confined aqueous compartments by raster image correlation spectroscopy (CADRICS). Lab Chip 2016, 16, 4666–4676. [Google Scholar] [CrossRef] [PubMed]
- Arrabito, G.; Cavaleri, F.; Porchetta, A.; Ricci, F.; Vetri, V.; Leone, M.; Pignataro, B. Printing Life-Inspired Subcellular Scale Compartments with Autonomous Molecularly Crowded Confinement. Adv. Biosyst. 2019, 3, 1900023. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.; Won, Y.; Hong, H.; Kim, Y.; Song, H.; Pyun, J.-C.; Cho, Y.S.; Ryu, W.; Moon, J. Insertion of Vertically Aligned Nanowires into Living Cells by Inkjet Printing of Cells. Small 2016, 12, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, S.; Weckhuysen, B.M.; Pettignano, A.; Pignataro, B. Multi-doped Brookite-Prevalent TiO2 Photocatalyst with Enhanced Activity in the Visible Light. Catal. Lett. 2018, 148, 2459–2471. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noman, M.T.; Ahraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 3262–3291. [Google Scholar] [CrossRef] [PubMed]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium Dioxide in the Service of the Biomedical Revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The Effect of Electrolyte Composition on the Fabrication of Self-Organized Titanium Oxide Nanotube Arrays by Anodic Oxidation. J. Mater. Res. 2005, 20, 230–236. [Google Scholar] [CrossRef]
- Nian, J.-N.; Teng, H. Hydrothermal Synthesis of Single-Crystalline Anatase TiO2 Nanorods with Nanotubes as the Precursor. J. Phys. Chem. B 2006, 110, 4193–4198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Byun, H.; Kumar, S.; Perikamana, M.; Lee, S. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1801106. [Google Scholar] [CrossRef] [PubMed]
- Goriainov, V.; Hulsart-billstrom, G.; Sjostrom, T.; Dunlop, D.G.; Su, B. Harnessing Nanotopography to Enhance Osseointegration of Clinical Orthopedic Titanium Implants—An in Vitro and in Vivo Analysis. Front Bioeng. Biotechnol. 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar] [PubMed] [Green Version]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.C.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, T.; Mcnamara, L.E.; Meek, R.M.D.; Dalby, M.J.; Su, B. 2D and 3D Nanopatterning of Titanium for Enhancing Osteoinduction of Stem Cells at Implant Surfaces. Adv Health Mater. 2013, 2, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, L.E.; Sjöström, T.; Burgess, K.E.V.; Kim, J.J.W.; Liu, E.; Gordonov, S.; Moghe, P.V.; Meek, R.M.D.; Oreffo, R.O.C.; Su, B.; et al. Biomaterials Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials 2011, 32, 7403–7410. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; Von Der Mark, K. Synergistic control of mesenchymal stem cell differentiation by nanoscale surface geometry and immobilized growth factors on TiO2 nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, O.; Gan, Y.; Li, Q.; Zhou, T.; Xi, W. Effect of titanium implants with coatings of different pore sizes on adhesion and osteogenic differentiation of BMSCs Effect of titanium implants with coatings of different pore sizes on adhesion and. Artif. Cells Nanomed. Biotechnol. 2019, 47, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Song, Y.; Ma, A.; Li, C. Surface Immobilization of TiO2 Nanotubes with Bone Morphogenetic Protein-2 Synergistically Enhances Initial Preosteoblast Adhesion and Osseointegration. Biomed Res. Int. 2019, 2019, 5697250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Ruan, M.; Xiao, J.; Zhang, Z.; Chen, C.; Zhang, W.; Cao, Y.; He, R.; Liu, Y.; Chen, Y. TiO2 Nanorod Arrays with Mesoscopic Micro-Nano Interfaces for in Situ Regulation of Cell Morphology and Nucleus Deformation. ACS Appl. Mater. Interfaces 2018, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar]
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale Topography on Black Titanium Imparts Multi-biofunctional Properties for Orthopedic Applications. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-cruz, D.; González, M.U.; Tien-street, W.; Fernández-castro, M.; Vernet-crua, A.; Fernández-martínez, I.; Martínez, L.; Huttel, Y.; Webster, T.J.; García-martín, J.M. Synergic antibacterial coatings combining titanium nanocolumns and tellurium nanorods. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and bactericidal surfaces from hydrothermal titania nanowires on titanium substrates. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Khanh Truong, V.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nataraj, N.; Anjusree, G.S.; Madhavan, A.A.; Priyanka, P.; Sankar, D.; Nisha, N.; Lakshmi, S.V.; Jayakumar, R.; Balakrishnan, A.; Biswas, R. Synthesis and Anti-Staphylococcal Activity of TiO2 Nanoparticles and Nanowires in. J. Biomed. Nanotechnol. 2014, 10, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Kumar, A.; Ram, M.K. Nanostructured photocatalysis in the visible spectrum for the decontamination of air and water. Int. Mater. Rev. 2018, 63, 257–282. [Google Scholar] [CrossRef]
- Zhao, Q.; Wen, W.; Xia, Y.; Wu, J. Photocatalytic activity of TiO2 nanorods, nanowires and nanoflowers filled with TiO2 nanoparticles. Thin Solid Films 2018, 648, 103–107. [Google Scholar] [CrossRef]
- Kamat, P.V. TiO2 Nanostructures: Recent Physical Chemistry Advances. J. Phys. Chem. C 2012, 116, 11849–11851. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Barakat, N.A.M.; Pandeya, D.R.; Hong, S.T.; Khil, M.-S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Aboelzahab, A.; Azad, A.; Dolan, S.; Goel, V. Mitigation of Staphylococcus aureus -Mediated Surgical Site Infections with IR Photoactivated TiO2 coatings on Ti Implants. Adv. Healthc. Mater. 2012, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Munisparan, T.; Chia, E.; Yang, Y.; Paramasivam, R.; Dahlan, N.A.; Pushpamalar, J. Optimisation of preparation conditions for Ti nanowires and suitability as an antibacterial material. IET Nanobiotechnol. 2018, 12, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, F.F.; Liu, H.Q.; Wang, Z.X.; Zhang, Z.T.; Wang, Y.; Cui, H.; Liu, W.; Zhao, X.Z.; Sun, Z.J.; et al. Efficient Capture and High Activity Release of Circulating Tumor Cells by Using TiO2 Nanorod Arrays Coated with Soluble MnO2 Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 16327–16334. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.R.; Sharma, A.; Nozari, A.; Subramaniam, R.; Sharma, T.L. and H.S. Nanowired Drug Delivery to Enhance Neuroprotection in Spinal Cord Injury. CNS Neurol. Disord. Drug Targets 2012, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Menon, P.; F Muresanu, D.; Ozkizilcik, A.; Ryan Tian, Z.; V Lafuente, J.; S Sharma, H. Nanowired Drug Delivery Across the Blood-Brain Barrier in Central Nervous System Injury and Repair. CNS Neurol. Disord. Drug Targets 2016, 15, 1092–1117. [Google Scholar] [CrossRef] [PubMed]
- S Sharma, H.; Feng, L.; V Lafuente, J.; F Muresanu, D.; R Tian, Z.; Patnaik, R.; Sharma, A. TiO2-Nanowired Delivery of Mesenchymal Stem Cells Thwarts Diabetes-Induced Exacerbation of Brain Pathology in Heat Stroke: An Experimental Study in the Rat Using Morphological and Biochemical Approaches. CNS Neurol. Disord. Drug Targets 2015, 14, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Muresanu, D.F.; Lafuente, J.V. Co-Administration of TiO2 Nanowired Mesenchymal Stem Cells with Cerebrolysin Potentiates Neprilysin Level and Reduces Brain Pathology in Alzheimer ’s Disease. Mol. Neurobiol. 2018, 55, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Shrestha, S.; Raj, E.; Yeon, J.; Kim, B.; Hee, C.; Sang, C. π-Conjugated polyaniline-assisted flexible titania nanotubes with controlled surface morphology as regenerative medicine in nerve cell growth. Chem. Eng. J. 2019, 360, 701–713. [Google Scholar]
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Wang, Z.; Xue, T.; Jiang, M. Nanowire arrays restore vision in blind mice. Nat. Commun. 2018, 9, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronzani, C.; Cottineau, T.; Gonzalez-Valls, I.; Keller, V.; Picaud, S.; Keller, N.; Roux, M.J. High-Frequency Stimulation of Normal and Blind Mouse Retinas Using TiO2 Nanotubes. Adv. Funct. Mater. 2018, 28, 1804639. [Google Scholar] [CrossRef]
- Bhat, S.S.; Qurashi, A.; Khanday, F.A. ZnO nanostructures based biosensors for cancer and infectious disease applications: Perspectives, prospects and promises. TrAC Trends Anal. Chem. 2017, 86, 1–13. [Google Scholar] [CrossRef]
- Niepelt, R.; Schroder, U.; Sommerfeld, J.; Slowik, I.; Rudolph, B.; Moller, R.; Seise, B.; Csaki, A.; Fritzsche, W.; Ronning, C. Biofunctionalization of zinc oxide nanowires for DNA sensory applications. Nanoscale Res. Lett. 2011, 6, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragalà, M.E.; Cacciotti, I.; Aleeva, Y.; Lo Nigro, R.; Bianco, A.; Malandrino, G.; Spinella, C.; Pezzotti, G.; Gusmano, G. Core–shell Zn-doped TiO2–ZnO nanofibers fabricated via a combination of electrospinning and metal–organic chemical vapour deposition. CrystEngComm 2010, 12, 3858–3865. [Google Scholar] [CrossRef]
- Fragalà, M.E.; Aleeva, Y.; Malandrino, G. Effects of Metal-Organic Chemical Vapour Deposition grown seed layer on the fabrication of well aligned ZnO nanorods by Chemical Bath Deposition. Thin Solid Films 2011, 519, 7694–7701. [Google Scholar] [CrossRef] [Green Version]
- Errico, V.; Arrabito, G.; Plant, S.R.; Medaglia, P.G.; Palmer, R.E.; Falconi, C. Chromium inhibition and size-selected Au nanocluster catalysis for the solution growth of low-density ZnO nanowires. Sci. Rep. 2015, 5, 12336. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Song, M.; Kim, J.; Kim, Y.; Song, H.; Yu, Y. Effect of Ammonia Water on the Morphology of Monoethanolamine-Assisted Sonochemicaly Synthesized ZnO Nanostructures. Nanosci. Nanotechnol. 2012, 12, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, R.; Yu, M.; Bai, F.; Li, C.; Wang, Z.L. Cellular Level Biocompatibility and Biosafety of ZnO Nanowires. J. Phys. Chem. C 2008, 112, 20114–20117. [Google Scholar] [CrossRef] [Green Version]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc Oxide Protects Cultured Enterocytes from the Damage Induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ågren, M.; Mirastschijski, U. The release of zinc ions from and cytocompatibility of two zinc oxide dressings. J. Wound Care 2004, 13, 367–369. [Google Scholar] [PubMed]
- Zong, X.; Zhu, R. Zinc oxide nanorod field effect transistor for long-time cellular force measurement. Sci. Rep. 2017, 7, 43661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Chen, Y.W.; Hsiao, C.S.; Chen, S.Y. Electrically responsive ZnO nanotubes for controlled release of biomolecules. Ceram. Int. 2017, 43, S802–S806. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, Y.-J.; Yeom, J.; Jeon, J.H.; Yi, G.-C.; Je, J.H.; Hahn, S.K. The Topographic Effect of Zinc Oxide Nanoflowers on Osteoblast Growth and Osseointegration. Adv. Mater. 2010, 22, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lin, Q.; Sun, H.; Chen, D.; Wu, Q.; Chen, X.; Li, S. Cytotoxic effects of ZnO hierarchical architectures on RSC96 Schwann cells. Nanoscale Res. Lett. 2012, 7, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanni, E.; De Palma, S.; Chandraiahgari, C.R.; De Bellis, G.; Cialfi, S.; Talora, C.; Palleschi, C.; Sarto, M.S.; Uccelletti, D.; Mancini, P. In vitro toxicity studies of zinc oxide nano- and microrods on mammalian cells: A comparative analysis. Mater. Lett. 2016, 179, 90–94. [Google Scholar] [CrossRef]
- Wang, Y.; Prox, J.D.; Yan, B.; Wu, Y.; Argall, A.D.; Guo, L. Zinc oxide nanorod array as an inhibitory biointerface. MRS Commun. 2018, 8, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2017, 79, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, B.S.; Hicks, B.; Chancellor, T.F., Jr.; Chu, B.H.; Wang, H.-T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chu, B.H.; Chen, K.-H.; Ren, F.; Lele, T.P. Randomly oriented, upright SiO2 coated nanorods for reduced adhesion of mammalian cells. Biomaterials 2009, 30, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, T.D.; Dolgova, N.V.; Chu, B.H.; Lee, J.; Wong, J.; Lele, T.P.; Ren, F.; Keselowsky, B.G. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials 2010, 31, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.H.; Kulkarni, J.; Motskin, M.; Goode, A.; Winship, P.; Skepper, J.N.; Ryan, M.P.; Porter, A.E. pH-Dependent Toxicity of High Aspect Ratio ZnO Nanowires in Macrophages Due to Intracellular Dissolution. ACS Nano 2010, 4, 6767–6779. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Moztarzadeh, F.; Safavi, M. A comparative study on the shape-dependent biological activity of nanostructured zinc oxide. Ceram. Int. 2019, 45, 1179–1188. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Quadri, F.; Prox, D.J.; Guo, L. Cytotoxicity of ZnO Nanowire Arrays on Excitable Cells. Nanomaterials 2017, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Raos, B.J.; Maddah, M.; Graham, E.S.; Plank, N.O.V.; Unsworth, C.P. ZnO Nanowire Florets Promote the Growth of Human Neurons. Materialia 2019, 9, 100577. [Google Scholar] [CrossRef]
- Errico, V.; Arrabito, G.; Fornetti, E.; Fuoco, C.; Testa, S.; Saggio, G.; Rufini, S.; Cannata, S.; Desideri, A.; Falconi, C.; et al. High-Density ZnO Nanowires as a Reversible Myogenic–Differentiation Switch. ACS Appl. Mater. Interfaces 2018, 10, 14097–14107. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, K.; Prabhu, R.; Pollard, P. Investigations on surface wettability of ZnO nanowires using UV LEDs for biosensing applications. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 12033. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Ekblad, T.; Yakimova, R.; Hultman, L. Surface morphology effects on the light-controlled wettability of ZnO nanostructures. Appl. Surf. Sci. 2012, 258, 8146–8152. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Okyay, T.O.; Bala, R.K.; Nguyen, H.N.; Atalay, R.; Bayam, Y.; Rodrigues, D.F. Antibacterial properties and mechanisms of toxicity of sonochemically grown ZnO nanorods. RSC Adv. 2015, 5, 2568–2575. [Google Scholar] [CrossRef]
- Yi, G.; Yuan, Y.; Li, X.; Zhang, Y. ZnO Nanopillar Coated Surfaces with Substrate-Dependent Superbactericidal Property. Small 2018, 14, 1703159. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.; Chang, L.; Zheng, Y. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, C.; Petrilli, L.L.; Cannata, S.; Gargioli, C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J. Orthop. Surg. Res. 2016, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-S.; Yoon, S.Y.; Park, J.S.; Lee, J.S. Deflection induced cellular focal adhesion and anisotropic growth on vertically aligned silicon nanowires with differing elasticity. NPG Asia Mater. 2016, 8, e249. [Google Scholar] [CrossRef] [Green Version]
- Onesto, V.; Villani, M.; Narducci, R.; Malara, N.; Imbrogno, A.; Allione, M.; Costa, N. Cortical-like mini-columns of neuronal cells on zinc oxide nanowire surfaces. Sci. Rep. 2019, 9, 4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacchioli, A.; Ravanetti, F.; Alinovi, R.; Pinelli, S.; Rossi, F.; Negri, M.; Bedogni, E.; Campanini, M.; Galetti, M.; Goldoni, M.; et al. Cytocompatibility and Cellular Internalization Mechanisms of SiC/SiO2 Nanowires. Nano Lett. 2014, 14, 4368–4375. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Luebben, M.; Ma, Z.; Dunin-borkowski, R.; Pirri, C.F.; Waser, R.; Ricciardi, C.; Valov, I.; Boarino, L. Self-limited single nanowire systems combining all-in-one memristive and neuromorphic functionalities. Nat. Commun. 2018, 9, 5151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Cho, H.A.; Lee, J.-W.; Ham, W.S.; Park, B.C.; Cho, N.-H.; Kim, Y.K. Efficient intracellular delivery of biomacromolecules employing clusters of zinc oxide nanowires. Nanoscale 2017, 9, 15371–15378. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shin, J.B.; Park, B.C.; Lee, J.-W.; Byun, S.W.; Jang, N.-Y.; Kim, Y.J.; Kim, Y.; Kim, Y.K.; Cho, N.-H. Application of radially grown ZnO nanowires on poly-l-lactide microfibers complexed with a tumor antigen for cancer immunotherapy. Nanoscale 2019, 11, 4591–4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, S.; Salice, P.; Menna, E.; Pignataro, B. Carbon nanotubes and organic solar cells. Energy Environ. Sci. 2012, 5, 5919–5940. [Google Scholar] [CrossRef]
- Salice, P.; Sartorio, C.; Burlini, A.; Improta, R.; Pignataro, B.; Menna, E. On the trade-off between processability and opto-electronic properties of single wall carbon nanotube derivatives in thin film heterojunctions. J. Mater. Chem. C 2015, 3, 303–312. [Google Scholar] [CrossRef]
- Lee, J.; Llerena Zambrano, B.; Woo, J.; Yoon, K.; Lee, T. Recent Advances in 1D Stretchable Electrodes and Devices for Textile and Wearable Electronics: Materials, Fabrications, and Applications. Adv. Mater. 2020, 32, 1902532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Kalmykov, A.; Johnson, N.; Cohen-Karni, T. Bioelectronics with nanocarbons. J. Mater. Chem. B 2018, 6, 7159–7178. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, X.; Xu, W.; Luo, W.; Li, M.; Chu, F.; Xu, L.; Cao, A.; Guan, J.; Tang, S.; et al. Stretchable Transparent Electrode Arrays for Simultaneous Electrical and Optical Interrogation of Neural Circuits in Vivo. Nano Lett. 2018, 18, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.S.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L.; et al. Carbon Nanotubes Instruct Physiological Growth and Functionally Mature Syncytia: Nongenetic Engineering of Cardiac Myocytes. ACS Nano 2013, 7, 5746–5756. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.L.; Agarwala, S.; Tan, Y.J.; Yeong, W.Y. A low cost and flexible carbon nanotube pH sensor fabricated using aerosol jet technology for live cell applications. Sens. Actuators B Chem. 2018, 260, 227–235. [Google Scholar] [CrossRef]
- Shin, S.R.; Migliori, B.; Miccoli, B.; Li, Y.; Mostafalu, P.; Seo, J.; Mandla, S.; Enrico, A.; Antona, S.; Sabarish, R.; et al. Electrically Driven Microengineered Bioinspired Soft Robots. Adv. Mater. 2018, 30, 1704189. [Google Scholar]
- Scuratti, F.; Bonacchini, G.E.; Bossio, C.; Salazar-Rios, J.M.; Talsma, W.; Loi, M.A.; Antognazza, M.R.; Caironi, M. Real-Time Monitoring of Cellular Cultures with Electrolyte-Gated Carbon Nanotube Transistors. ACS Appl. Mater. Interfaces 2019, 11, 37966–37972. [Google Scholar] [CrossRef] [PubMed]
- Rago, I.; Rauti, R.; Bevilacqua, M.; Calaresu, I.; Pozzato, A.; Cibinel, M.; Dalmiglio, M.; Tavagnacco, C.; Goldoni, A.; Scaini, D. Carbon Nanotubes, Directly Grown on Supporting Surfaces, Improve Neuronal Activity in Hippocampal Neuronal Networks. Adv. Biosyst. 2019, 3, 1800286. [Google Scholar] [CrossRef]
- Stout, D.A.; Webster, T.J. Carbon nanotubes for stem cell control. Mater. Today 2012, 15, 312–318. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2006, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Fadel, T.R.; Sharp, F.A.; Vudattu, N.; Ragheb, R.; Garyu, J.; Kim, D.; Hong, E.; Li, N.; Haller, G.L.; Pfefferle, L.D.; et al. A carbon nanotube–polymer composite for T-cell therapy. Nat. Nanotechnol. 2014, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- García-Hevia, L.; Fernández, F.; Grávalos, C.; García, A.; Villegas, J.C.; Fanarraga, M.L. Nanotube interactions with microtubules: Implications for cancer medicine. Nanomedicine 2014, 9, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Farajpour, A.; Rastgoo, A. Influence of carbon nanotubes on the buckling of microtubule bundles in viscoelastic cytoplasm using nonlocal strain gradient theory. Results Phys. 2017, 7, 1367–1375. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, L.; Valiente, R.; Gonzalez, J.; Villegas, J.C.; Fanarraga, M.L. Multiwalled Carbon Nanotubes Display Microtubule Biomimetic Properties in Vivo, Enhancing Microtubule Assembly and Stabilization. ACS Nano 2012, 6, 6614–6625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Qian, Z. Nanostructured Surface Modification to Bone Implants for Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 628–648. [Google Scholar] [CrossRef] [PubMed]
- Peretz, S.; Regev, O. Carbon nanotubes as nanocarriers in medicine. Curr. Opin. Colloid Interface Sci. 2012, 17, 360–368. [Google Scholar] [CrossRef]
- Gangrade, A.; Mandal, B.B. An Injectable Carbon Nanotube Impregnated Silk Based Multifunctional Hydrogel for Localized Targeted and On Demand Anticancer Drug Delivery. ACS Biomater. Sci. Eng. 2019, 5, 2365–2381. [Google Scholar] [CrossRef]
- You, Y.; Wang, N.; He, L.; Shi, C.; Zhang, D.; Liu, Y.; Luo, L.; Chen, T. Designing dual-functionalized carbon nanotubes with high blood–brain-barrier permeability for precise orthotopic glioma therapy. Dalt. Trans. 2019, 48, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S.; Langer, R. Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 2008, 63, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignataro, B.; Conte, E.; Scandurra, A.; Marletta, G. Improved cell adhesion to ion beam-irradiated polymer surfaces. Biomaterials 1997, 18, 1461–1470. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M.; Ahmad, S.R. Fabrication of polymeric biomaterials: A strategy for tissue engineering and medical devices. J. Mater. Chem. B 2015, 3, 8224–8249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Jin, G.; Li, L.; Li, K.; Srinivasan, M.; Ramakrishna, S.; Chen, J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem. Soc. Rev. 2016, 45, 1225–1241. [Google Scholar] [PubMed]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Tran, H.D.; Li, D.; Kaner, R.B. One-dimensional conducting polymer nanostructures: Bulk synthesis and applications. Adv. Mater. 2009, 21, 1487–1499. [Google Scholar] [CrossRef]
- Dolatshahi-Pirouz, A.; Kolind, K.; Pennisi, C.P.; Duroux, M.; Zachar, V.; Foss, M.; Besenbacher, F. Synthesis of Nano- and Micro-Scale Topographies by Combining Colloidal Lithography and Glancing Angle Deposition (GLAD). Adv. Eng. Mater. 2015, 17, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; McMurray, R.J.; Burgess, K.V.; Alakpa, E.V.; Reynolds, P.M.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Gadegaard, N.; Dalby, M.J. Using nanotopography and metabolomics to identify biochemical effectors of multipotency. ACS Nano 2012, 6, 10239–10249. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Yang, S.; Chen, Y.; Chen, K. Phosphate Composite Fibrous Mats for. Polymers 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont-Gillain, C.C. Understanding and controlling type I collagen adsorption and assembly at interfaces, and application to cell engineering. Colloids Surf. B 2014, 124, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, V.; Zito, G.; Arrabito, G.; Cataldo, S.; Scopelliti, M.; Giordano, C.; Vetri, V.; Pignataro, B. Aqueous Processed Biopolymer Interfaces for Single-Cell Microarrays. ACS Biomater. Sci. Eng. 2020, 6, 3174–3186. [Google Scholar] [CrossRef]
- Liu, G.Y.; Agarwal, R.; Ko, K.R.; Ruthven, M.; Sarhan, H.T.; Frampton, J.P. Templated Assembly of Collagen Fibers Directs Cell Growth in 2D and 3D. Sci. Rep. 2017, 7, 9628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, B.; Zhang, H.; Yu, Z.; Yuan, H.; Wang, X.; Zhang, Y. Fabrication of high performance silk fibroin fibers via stable jet electrospinning for potential use in anisotropic tissue regeneration. J. Mater. Chem. B 2018, 6, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L.; Zussman, E.; Xu, H. Electrospinning of Nanofibers from Polymer Solutions and Melts. Adv. Appl. Mech. 2007, 41, 343–346. [Google Scholar]
- Sun, F.; Chen, J.; Jin, S.; Wang, J.; Man, Y.; Li, J.; Zou, Q.; Li, Y.; Zuo, Y. Development of biomimetic trilayer fibrous membranes for guided bone regeneration. J. Mater. Chem. B 2019, 7, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, H.; McCarthy, A.; Yan, Z.; Kim, H.J.; Carlson, M.A.; Xia, Y.; Xie, J. Three-Dimensional Objects Consisting of Hierarchically Assembled Nanofibers with Controlled Alignments for Regenerative Medicine. Nano Lett. 2019, 19, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Omidinia-Anarkoli, A.; Rimal, R.; Chandorkar, Y.; Gehlen, D.B.; Rose, J.C.; Rahimi, K.; Haraszti, T.; De Laporte, L. Solvent-Induced Nanotopographies of Single Microfibers Regulate Cell Mechanotransduction. ACS Appl. Mater. Interfaces 2019, 11, 7671–7685. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Asheghali, D.; Blevins, B.; Timsina, R.; Esworthy, T.; Zhou, X.; Cui, H.; Hann, S.Y.; Qiu, X.; Tokarev, A.; et al. Touch-Spun Nanofibers for Nerve Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, R.; Wang, P.P.; Jian, J.; Jiang, X.L.; Yan, C.; Lin, X.; Wu, L.; Chen, G.Q.; Wu, Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials 2012, 33, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, P.K.; Metwally, S.; Karbowniczek, J.E.; Marzec, M.M.; Stodolak-Zych, E.; Gruszczyński, A.; Bernasik, A.; Stachewicz, U. Surface-Potential-Controlled Cell Proliferation and Collagen Mineralization on Electrospun Polyvinylidene Fluoride (PVDF) Fiber Scaffolds for Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 582–593. [Google Scholar] [CrossRef]
- Guo, T.; Ringel, J.P.; Lim, C.G.; Bracaglia, L.G.; Noshin, M.; Baker, H.B.; Powell, D.A.; Fisher, J.P. Three dimensional extrusion printing induces polymer molecule alignment and cell organization within engineered cartilage. J. Biomed. Mater. Res. A 2018, 106, 2190. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sharma, C.S.; Rath, S.N. Enhanced osteodifferentiation of MSC spheroids on patterned electrospun fiber mats—An advanced 3D double strategy for bone tissue regeneration. Mater. Sci. Eng. C 2019, 94, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Gu, Y.; Shen, X.; Zhang, Y.; Li, B.; Chen, L. Polydopamine-modified poly(l-lactic acid) nanofiber scaffolds immobilized with an osteogenic growth peptide for bone tissue regeneration. RSC Adv. 2019, 9, 11722–11736. [Google Scholar] [CrossRef] [Green Version]
- Kouhi, M.; Jayarama Reddy, V.; Fathi, M.; Shamanian, M.; Valipouri, A.; Ramakrishna, S. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/fibrinogen/bredigite nanofibrous membranes and their integration with osteoblasts for guided bone regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 1154. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, L.; Liu, Y.; Zheng, A.; Jiao, D.; Zeng, D.; Wang, X.; Kaplan, D.L.; Jiang, X. Functionalization of Silk Fibroin Electrospun Scaffolds via BMSC Affinity Peptide Grafting through Oxidative Self-Polymerization of Dopamine for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 8878–8895. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.L.; Valente, C.A.; Zanini, M.L.; Sgarioni, B.; Henrique, P.; Tondo, F.; Chagastelles, P.C.; Braga, J.; Campos, M.M.; Malmonge, A.; et al. Biocompatible PCL/PLGA/Polypyrrole Composites for Regenerating Nerves. Macromol. Symp. 2019, 383, 1800028. [Google Scholar] [CrossRef] [Green Version]
- Nekouian, S.; Sojoodi, M.; Nadri, S. Fabrication of conductive fibrous scaffold for photoreceptor differentiation of mesenchymal stem cell. J. Cell Physiol 2019, 234, 15800. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Huang, Y.; Wei, P.; Cai, Q.; Yang, X.; Zhong, W. Roles of electrical stimulation in promoting osteogenic differentiation of BMSCs on conductive fibers. J. Biomed. Mater. Res. A 2019, 107, 1443. [Google Scholar] [CrossRef] [PubMed]
- Hronik-Tupaj, M.; Kaplan, D.L. A Review of the Responses of Two- and Three-Dimensional Engineered Tissues to Electric Fields. Tissue Eng. Part B Rev. 2011, 18, 167–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Peng, Y.; Bohra, H.; Zou, J.; Ranjan, V.D.; Zhang, Y.; Zhang, Q.; Wang, M. Photoconductive Micro/Nanoscale Interfaces of a Semiconducting Polymer for Wireless Stimulation of Neuron-Like Cells. ACS Appl. Mater. Interfaces 2019, 11, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Sekine, J.; Luo, S.; Wang, S.; Zhu, B.; Tseng, H. Functionalized Conducting Polymer Nanodots for Enhanced Cell Capturing: The Synergistic Effect of Capture Agents and Nanostructures. Adv. Mater. 2011, 23, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, L.; Liu, H.; Yang, G.; Zhang, P.; Han, D.; Wang, S.; Jiang, L. Bio-inspired soft polystyrene nanotube substrate for rapid and highly efficient breast cancer-cell capture. NPG Asia Mater. 2013, 5, e63. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.S.; Luo, S.C.; Hou, S.; Zhu, B.; Sekine, J.; Kuo, C.W.; Chueh, D.Y.; Yu, H.H.; Tseng, H.R.; Chen, P. 3D bioelectronic interface: Capturing circulating tumor cells onto conducting polymer-based micro/nanorod arrays with chemical and topographical control. Small 2014, 10, 3012–3017. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Ho, B.-C.; Juang, R.-S.; Hsiao, Y.-S.; Venkata Ram Naidu, R.; Kuo, C.-W.; You, Y.-W.; Shyue, J.-J.; Fang, J.-T.; Chen, P. Poly(3,4-ethylenedioxythiophene)-Based Nanofiber Mats as an Organic Bioelectronic Platform for Programming Multiple Capture/Release Cycles of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2017, 9, 30329–30342. [Google Scholar] [CrossRef] [PubMed]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragon fly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; YEE, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, C.B.; Kim, J.; Schlesinger, E.B.; Chirra, H.D.; Desai, T.A. Fabrication of Micropatterned Polymeric Nanowire Arrays for High-Resolution Reagent Localization and Topographical Cellular Control. Nano Lett. 2015, 15, 1540–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Kang, H.; Lee, C.; Hwang, S.H.; Jang, J. Aqueous Synthesis of Silver Nanoparticle Embedded Cationic Polymer Nanofibers and Their Antibacterial Activity. ACS Appl. Mater. Interfaces 2012, 4, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, W.; Zhang, L.; Zhong, S.; Yu, D. Electrospinning of PAN/AgNPs nanofiber membrane with antibacterial properties. J. Mater. Res. 2019, 34, 1–9. [Google Scholar]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nano fi bers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goettems, S.; Alberto, J.; Costa, V.; Greque, M.; Morais, D. Development of electrospun nano fi bers containing chitosan/PEO blend and phenolic compounds with antibacterial activity. Int. J. Biol. Macromol. 2018, 117, 800–806. [Google Scholar]
- Koushki, P.; Bahrami, S.H.; Ranjbar-mohammadi, M. Coaxial nanofibers from poly(caprolactone)/poly (vinyl alcohol)/Thyme and their antibacterial properties. J. Ind. Text. 2018, 47, 834. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Pol, J.; Slukin, P.V.; Ignatov, S.G.; Gloushankova, N.A.; Zají, L.; Shtansky, D.V.; Manakhov, A. Antibacterial biocompatible PCL nanofibers modified by COOH-anhydride plasma polymers and gentamicin immobilization. Mater. Des. 2018, 153, 60–70. [Google Scholar] [CrossRef]
- Akia, M.; Rodriguez, C.; Materon, L.; Gilkerson, R.; Lozano, K. Antibacterial activity of polymeric nanofiber membranes impregnated with Texas sour orange juice. Eur. Polym. J. 2019, 115, 1–5. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; Mcdaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 35386–35394. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Arrabito, G. Hybrid, multiplexed, functional DNA nanotechnology for bioanalysis. Analyst 2015, 140, 5821–5848. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Park, S.H.; Reif, J.H.; LaBean, T.H. DNA nanotubes self-assembled from triple-crossover tiles as templates for conductive nanowires. Proc. Natl. Acad. Sci. USA 2004, 101, 717–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Park, S.H.; Finkelstein, G.; Reif, J.H.; LaBean, T.H. DNA-Templated Self-Assembly of Protein Arrays and Highly Conductive Nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Shimron, S.; Wang, F.; Orbach, R.; Willner, I. Amplified detection of DNA through the enzyme-free autonomous assembly of hemin/G-quadruplex DNAzyme nanowires. Anal. Chem. 2012, 84, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Dai, J.; Dong, G.; Shi, H.; Wang, F.; Qiu, Y.; Liao, R.; Zhou, C.; Guo, Y.; Xiao, D. Self-Replication-Assisted Rapid Preparation of DNA Nanowires at Room Temperature and Its Biosensing Application. Anal. Chem. 2019, 91, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, C.B. High-temperature self-assembly of peptides into vertically well-aligned nanowires by aniline vapor. Adv. Mater. 2008, 20, 3754–3758. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, M.; Park, C.B. Bone-like peptide/hydroxyapatite nanocomposites assembled with multi-level hierarchical structures. Soft Matter 2011, 7, 7201–7206. [Google Scholar] [CrossRef]
- Sasso, L.; Vedarethinam, I.; Emnéus, J.; Svendsen, W.E.; Castillo-Leóon, J. Self-Assembled Diphenylalanine Nanowires for Cellular Studies and Sensor Applications. J. Nanosci. Nanotechnol. 2012, 12, 3077–3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Zhang, A.; You, S.S.; Lieber, C.M. Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Lett. 2016, 16, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, R.; Tian, L.; Soleimani, M.; Ramakrishna, S.; Naderi-Manesh, H. Peptide modified nanofibrous scaffold promotes human mesenchymal stem cell proliferation and long-term passaging. Mater. Sci. Eng. C 2017, 84, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Silantyeva, E.A.; Nasir, W.; Carpenter, J.; Manahan, O.; Becker, M.L.; Willits, R.K. Accelerated neural differentiation of mouse embryonic stem cells on aligned GYIGSR-functionalized nanofibers. Acta Biomater. 2018, 75, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Maeda, Y.; Mutter, A.C.; Matsunaga, T.; Xu, Y.; Matsui, H. Three-dimensional directed self-assembly of peptide nanowires into micrometer-sized crystalline cubes with nanoparticle joints. Angew. Chem. Int. Ed. 2010, 49, 8375–8378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, D.A.; Cheng, E.Y.; Guler, M.O.; Lee, L.K.; Donovan, J.L.; Claussen, R.C.; Stupp, S.I. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J. Biomed. Mater. Res. A 2006, 78, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Arizmendi, N.; Kulka, M.; Unsworth, L.D. The Spontaneous Adhesion of BMMC onto Self-Assembled Peptide Nanoscaffold without Activation Inhibits Its lgE-Mediated Degranulation. Adv. Healthc. Mater. 2017, 6, 1700334. [Google Scholar] [CrossRef] [PubMed]
- Somaa, F.A.; Wang, T.; Niclis, J.C.; Nisbet, D.R.; Thompson, L.H.; Parish, C.L.; Somaa, F.A.; Wang, T.; Niclis, J.C.; Bruggeman, K.F.; et al. Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Promote Functional Repair in a Model of Stroke Resource Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Pro. Cell Rep. 2017, 20, 1964–1977. [Google Scholar]
- Schilling, C.; Mack, T.; Lickfett, S.; Sieste, S.; Ruggeri, F.S.; Sneideris, T.; Dutta, A.; Bereau, T.; Naraghi, R.; Sinske, D.; et al. Sequence-Optimized Peptide Nanofibers as Growth Stimulators for Regeneration of Peripheral Neurons. Adv. Funct. Mater. 2019, 29, 1809112. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrabito, G.; Aleeva, Y.; Ferrara, V.; Prestopino, G.; Chiappara, C.; Pignataro, B. On the Interaction between 1D Materials and Living Cells. J. Funct. Biomater. 2020, 11, 40. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11020040
Arrabito G, Aleeva Y, Ferrara V, Prestopino G, Chiappara C, Pignataro B. On the Interaction between 1D Materials and Living Cells. Journal of Functional Biomaterials. 2020; 11(2):40. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11020040
Chicago/Turabian StyleArrabito, Giuseppe, Yana Aleeva, Vittorio Ferrara, Giuseppe Prestopino, Clara Chiappara, and Bruno Pignataro. 2020. "On the Interaction between 1D Materials and Living Cells" Journal of Functional Biomaterials 11, no. 2: 40. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11020040