Synthesis of Functional Silver Nanoparticles and Microparticles with Modifiers and Evaluation of Their Antimicrobial, Anticancer, and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Silver Nanoparticles and Microparticles
2.2. Characterization of Silver Nanoparticles and Microparticles
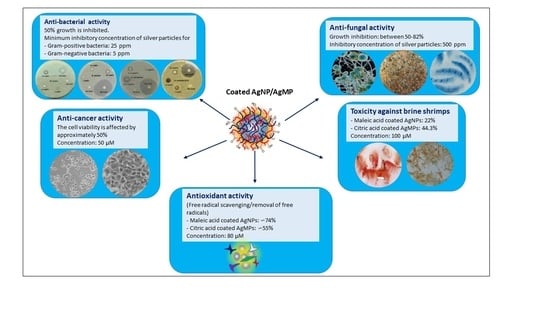
2.3. Antibacterial Assay
2.4. Antifungal Assay
2.5. Brine Shrimp Lethality Assay for Cytotoxicity Screening
2.6. MTT Assay on Human Cancer Cell Lines
2.7. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Silver Nanoparticles and Microparticles
3.1.1. Confirmation of Synthesis
3.1.2. Confirmation of Elemental Composition
3.1.3. Characterization for Structure and Size
3.1.4. Morphology by Scanning Electron Microscopy
3.2. Antibacterial Activity
3.3. Antifungal Activity
3.4. Cytotoxicity by Brine Shrimp Lethality and MTT Assay In Vitro
3.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed. 2003, 42, 2350–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Zhai, A. Preparation of microsized silver crystals with different morphologies by a wet-chemical method. Rare Met. 2010, 29, 407–412. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.-S.; Li, Y.; Wong, C. Surface functionalized silver nanoparticles for ultrahigh conductive polymer composites. Chem. Mater. 2006, 18, 2969–2973. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.-Y.; Takeda, Y.; Kondow, T.; Sawabe, H. Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation. J. Phys. Chem. B 2000, 104, 8333–8337. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Panáček, A.; Kvitek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bocate, K.P.; Reis, G.F.; De Souza, P.C.; Junior, A.G.O.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; De Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mussin, J.E.; Roldan, M.V.; Rojas, F.; Sosa, M.L.A.; Pellegri, N.; Giusiano, G. Antifungal activity of silver nanoparticles in combination with ketoconazole against Malassezia furfur. AMB Express 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Mashkani, S.M.; Ramezani, M. Silver and silver oxide nanoparticles: Synthesis and characterization by thermal decomposition. Mater. Lett. 2014, 130, 259–262. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials (Basel) 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, M.; Vennemann, A.; Blaske, F.; Sperling, M.; Karst, U. Silver Nanoparticles in the Lung: Toxic Effects and Focal Accumulation of Silver in Remote Organs. Nanomaterials (Basel) 2017, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Xi, T. Distribution, translocation and accumulation of silver nanoparticles in rats. J. Nanosci. Nanotechnol. 2009, 9, 4924–4932. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D.; et al. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats. Part. Fibre Toxicol. 2013, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem. A Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly (vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Botero, L.E.; Zárate-Triviño, D.; Acevedo-Yepes, N.; Escobar, J.S.; Pérez, V.Z.; Riano, L.J.C. Synthesis and characterization of a silver nanoparticle-containing polymer composite with antimicrobial abilities for application in prosthetic and orthotic devices. Biomater. Res. 2020, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Eiden-Assmann, S.; Widoniak, J.; Maret, G. Synthesis and characterization of porous and nonporous monodisperse colloidal TiO2 particles. Chem. Mater. 2004, 16, 6–11. [Google Scholar] [CrossRef]
- Sondi, I.; Goia, D.V.; Matijević, E. Preparation of highly concentrated stable dispersions of uniform silver nanoparticles. J. Colloid Interface Sci. 2003, 260, 75–81. [Google Scholar] [CrossRef]
- Flores, C.Y.; Miñán, A.G.; Grillo, C.A.; Salvarezza, R.C.; Vericat, C.; Schilardi, P.L. Citrate-capped silver nanoparticles showing good bactericidal effect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl. Mater. Interfaces 2013, 5, 3149–3159. [Google Scholar] [CrossRef]
- Lee, S.; Na, K. Oleic acid conjugated polymeric photosensitizer for metastatic cancer targeting in photodynamic therapy. Biomater. Res. 2020, 24, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helaly, F.; El-Sawy, S.; Hashem, A.; Khattab, A.; Mourad, R. Synthesis and characterization of nanosilver-silicone hydrogel composites for inhibition of bacteria growth. Contact Lens Anterior Eye 2017, 40, 59–66. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.; Seo, S.W. In situ synthesis of silver nanoparticles on the surface of PDMS with high antibacterial activity and biosafety toward an implantable medical device. Nano Converg. 2017, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Samoilova, N.; Kurskaya, E.; Krayukhina, M.; Askadsky, A.; Yamskov, I. Copolymers of maleic acid and their amphiphilic derivatives as stabilizers of silver nanoparticles. J. Phys. Chem. B 2009, 113, 3395–3403. [Google Scholar] [CrossRef]
- Jiang, X.C.; Chen, C.Y.; Chen, W.M.; Yu, A.B. Role of citric acid in the formation of silver nanoplates through a synergistic reduction approach. Langmuir 2010, 26, 4400–4408. [Google Scholar] [CrossRef]
- Umadevi, M.; Bindhu, M.R.; Sathe, V. A Novel Synthesis of Malic Acid Capped Silver Nanoparticles using Solanum lycopersicums Fruit Extract. J. Mater. Sci. Technol. 2013, 29, 317–322. [Google Scholar] [CrossRef]
- Bing, A.; Cai, X.-H.; Wu, F.-S.; Wu, Y.-P. Preparation of micro-sized and uniform spherical Ag powders by novel wet-chemical method. Trans. Nonferrous Met. Soc. China 2010, 20, 1550–1554. [Google Scholar]
- Maqbool, Q.; Nazar, M.; Naz, S.; Hussain, T.; Jabeen, N.; Kausar, R.; Anwaar, S.; Abbas, F.; Jan, T. Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract. Int. J. Nanomed. 2016, 11, 5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dong, P.; Ha, C.H.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, M.; Ahmad, I.; Ismail, H.; Ahmed, S.; McKee, V.; Akhter, Z.; Poczai, P. Pharmacological, electrochemical and drug–DNA interaction aspects of tridentate Schiff bases and their triphenylphosphine nickel (II) complexes. Polyhedron 2017, 133, 270–278. [Google Scholar] [CrossRef]
- Kumar, C.M.K.; Yugandhar, P.; Savithramma, N. Biological synthesis of silver nanoparticles from Adansonia digitata L. fruit pulp extract, characterization, and its antimicrobial properties. J. Intercult. Ethnopharmacol. 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Evaluation of the antibacterial and antifungal properties of Phragmanthera capitata (Sprengel) Balle (Loranthaceae), a mistletoe growing on rubber tree, using the dilution techniques. Sci. World J. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.S.; Hussain, M.; Hanif, M.; Ali, S.; Qayyum, M.; Mirza, B. Di-and Triorganotin (IV) Esters of 3, 4-Methylenedioxyphenylpropenoic Acid: Synthesis, Spectroscopic Characterization and Biological Screening for Antimicrobial, Cytotoxic and Antitumor Activities. Chem. Biol. Drug Des. 2008, 71, 568–576. [Google Scholar] [CrossRef]
- Dilshad, E.; Zafar, S.; Ismail, H.; Waheed, M.T.; Cusido, R.M.; Palazon, J.; Poczai, P. Effect of rol genes on polyphenols biosynthesis in Artemisia annua and their effect on antioxidant and cytotoxic potential of the plant. Appl. Biochem. Biotechnol. 2016, 179, 1456–1468. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech. 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castañón, G.; Niño-Martínez, N.; Loyola-Rodríguez, J.; Patiño-Marín, N.; Martínez-Mendoza, J.; Ruiz, F. Synthesis of silver particles with different sizes and morphologies. Mater. Lett. 2009, 63, 1266–1268. [Google Scholar] [CrossRef]
- Khatoon, N.; Ahmad, R.; Sardar, M. Robust and fluorescent silver nanoparticles using Artemisia annua: Biosynthesis, characterization and antibacterial activity. Biochem. Eng. J. 2015, 102, 91–97. [Google Scholar] [CrossRef]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N.; Chauhan, S. Antioxidant and antibacterial potential of silver nanoparticles: Biogenic synthesis utilizing apple extract. J. Pharm. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Karunagaran, V.; Rajendran, K.; Sen, S. Optimization of biosynthesis of silver oxide nanoparticles and its anticancer activity. Int. J. Nanosci. 2017, 16, 1750018. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Rai, M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Tamayo, L.; Zapata, P.; Vejar, N.; Azocar, M.; Gulppi, M.; Zhou, X.; Thompson, G.; Rabagliati, F.; Páez, M.A. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C 2014, 40, 24–31. [Google Scholar] [CrossRef]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Surface Decontamination of Fruits and Vegetables Eaten Raw: A Review; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Ballal, N.V.; Yegneswaran, P.P.; Mala, K.; Bhat, K.S. In vitro antimicrobial activity of maleic acid and ethylenediaminetetraacetic acid on endodontic pathogens. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 696–700. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Hettiarachchy, N.; Johnson, M. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella gaminara. J. Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- In, Y.W.; Kim, J.J.; Kim, H.J.; Oh, S.W. Antimicrobial activities of acetic acid, citric acid and lactic acid against S higella species. J. Food Saf. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, M.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Periasamy, S.; Joo, H.-S.; Duong, A.C.; Bach, T.-H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, V. Quorum sensing in biofilms–how to destroy the bacterial citadels or their cohesion/power? Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Rolim, J.P.; Melo, M.A.S.; Guedes, S.F.; Albuquerque-Filho, F.B.; De Souza, J.R.; Nogueira, N.A.P.; Zanin, I.C.; Rodrigues, L.K.A. The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers. J. Photochem. Photobiol. B Biol. 2012, 106, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bury, N.R.; Wood, C.M. Mechanism of branchial apical silver uptake by rainbow trout is via the proton-coupled Na+ channel. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R1385–R1391. [Google Scholar] [CrossRef]
- Martinez-Castanon, G.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martinez-Mendoza, J.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Hassan, R.; El-Kadi, S.; Sand, M. Effect of some organic acids on some fungal growth and their toxins production. Int. J. Adv. Biol. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Singh, V.; Shamsi, S.; Fatma, A.; Mehta, B. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol. Res. Int. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Kokati Venkata, B.R. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, E.Z. Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: A green approach. J. Genet. Eng. Biotechnol. 2017, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.; Brzezinski, P. Topical malic acid in combination with citric acid: An option to treat recalcitrant warts. Dermatol. Ther. 2015, 28, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.-H.; Chen, C.-W.; Hsiao, Y.-P.; Hung, S.-J.; Chung, J.-G.; Yang, J.-H. Citric acid induces cell-cycle arrest and apoptosis of human immortalized keratinocyte cell line (HaCaT) via caspase-and mitochondrial-dependent signaling pathways. Anticancer Res. 2013, 33, 4411–4420. [Google Scholar] [PubMed]
- Ren, J.-G.; Seth, P.; Ye, H.; Jian-Guo, R.; Hanai, J.-I.; Husain, Z.; Sukhatme, V.P. Citrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathway. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kurt, A.; Erkose-Genc, G.; Uzun, M.; Emrence, Z.; Ustek, D.; Isik-Ozkol, G. The antifungal activity and cytotoxicity of silver containing denture base material. Niger. J. Clin. Pract. 2017, 20, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.-H.; Park, K.; Yi, J.; Ryu, D.-Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; De La Fonteyne-Blankestijn, L.J.; Verharen, H.W.; Briedé, J.J.; Van Loveren, H.; De Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Park, J.; Lim, D.-H.; Lim, H.-J.; Kwon, T.; Choi, J.-S.; Jeong, S.; Choi, I.-H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Das, S.; Choudhury, P.; Ghosh, S.; Baral, R.; Choudhuri, S.K. A novel approach of synthesizing and evaluating the anticancer potential of silver oxide nanoparticles in vitro. Chemotherapy 2017, 62, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef]
- Netchareonsirisuk, P.; Puthong, S.; Dubas, S.; Palaga, T.; Komolpis, K. Effect of capping agents on the cytotoxicity of silver nanoparticles in human normal and cancer skin cell lines. J. Nanopart. Res. 2016, 18, 322. [Google Scholar] [CrossRef]
- A Franco-Molina, M.; Mendoza-Gamboa, E.; A Sierra-Rivera, C.; A Gómez-Flores, R.; Zapata-Benavides, P.; Castillo-Tello, P.; Alcocer-González, J.M.; Miranda-Hernández, D.F.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 1. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Seligy, V.L.; Massarsky, A.; Moon, T.W.; Rippstein, P.; Tan, J.; Tayabali, A.F. Comparison of toxicity of uncoated and coated silver nanoparticles. In Proceedings of Journal of Physics: Conference Series; IOP Publishing: Redcliffe, UK, 2013; p. 012025. [Google Scholar]
- Jurašin, D.D.; Ćurlin, M.; Capjak, I.; Crnković, T.; Lovrić, M.; Babič, M.; Horák, D.; Vrček, I.V.; Gajović, S. Surface coating affects behavior of metallic nanoparticles in a biological environment. Beilstein J. Nanotechnol. 2016, 7, 246–262. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; Obot, I.; Ukpong, U. Green synthesis of silver nanoparticles using Artemisia annua and Sida acuta leaves extract and their antimicrobial, antioxidant and corrosion inhibition potentials. J. Mater. Environ. Sci. 2014, 5, 899–906. [Google Scholar]
- Kazemi, M.; Hadavi, E.; Hekmati, J. Effect of salicylic acid, malic acid, citric acid and sucrose on antioxidant activity, membrane stability and ACC-oxidase activity in relation to vase life of carnation cut flowers. J. Agric. Technol. 2012, 8, 2053–2063. [Google Scholar] [CrossRef] [Green Version]







| AgNPsConc. (ppm) | Zone of Inhibition (cm) ± S.E | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive Strains | Gram-negative Strains | |||||||||||||||||
| M. luteus | S. aureus | B. subtiles | A. tumefaciens | S. setubal | E. aerogenes | |||||||||||||
| M.A. | C.A. | C | M.A | C.A. | C | M.A | C.A. | C | M.A | C.A | C | M.A | C.A | C | M.A | C.A | C | |
| 100 | 1.25 ± 0.9 | 0.88 ± 0.5 | 0.4 ± 1.2 | 1.21 ± 0.75 | 0.63 ± 0.89 | - | 1.31 ± 0.23 | 0.74 ± 0.60 | - | 3.1 ± 1.33 | 2.01 ± 1.3 | 0.7 ± 1 | 1.66 ± 0.5 | 1.2 ± 1.3 | 0.54 ± 1.3 | 3.23 ± 0.34 | 2.4 ± 1.3 | 0.5 ± 0.9 |
| 50 | 0.98 ± 1.1 | 0.56 ± 1.21 | - | 0.68 ± 0.66 | - | - | 0.64 ± 1.15 | 0.52 ± 0.98 | - | 2.5 ± 1.55 | 0.85 ± 0.89 | - | 1.16 ± 1.1 | 0.66 ± 0.56 | - | 2.24 ± 1.3 | 1.4 ± 1.12 | - |
| 25 | 0.74 ± 1.7 | 0.42 ± 0.82 | - | 0.46 ± 1.3 | - | - | 0.4 ± 01.2 | - | - | 0.87 ± 0.78 | 0.54 ± 0.44 | - | 0.76 ± 0.98 | 0.45 ± 1.2 | - | 1.76 ± 0.78 | 0.72 ± 0.98 | - |
| 5 | - | - | - | - | - | - | - | - | - | 0.51 ± 1.2 | - | - | 0.51 ± 0.2 | - | - | 0.79 ± 1.2 | 0.54 ± 0.23 | - |
| 2.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| AgNO3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Negative Control (Distilled H2O) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Positive Control (Streptomycin) | 2.1 | 2.42 | 2.4 | 3.4 | 4.72 | 4.2 | ||||||||||||
| Source of Variation | Df | Sum-of-Squares | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Interaction | 9 | 3091 | 343.5 | 117.4 | <0.0001 | Yes |
| Types of particles | 3 | 14280 | 4761 | 1628 | <0.0001 | Yes |
| Concentration | 3 | 6649 | 2216 | 757.7 | <0.0001 | Yes |
| Residual | 32 | 93.60 | 2.925 |
| Source of Variation | Df | Sum-of-Squares | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Interaction | 12 | 2227 | 185.6 | 97.86 | <0.0001 | Yes |
| Types of particles | 3 | 22690 | 7562 | 3988 | <0.0001 | Yes |
| Concentration | 4 | 5420 | 1355 | 714.6 | <0.0001 | Yes |
| Residual | 40 | 75.84 | 1.896 |
| Source of Variation | Df | Sum-of-Squares | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Interaction | 12 | 1676 | 139.6 | 100.5 | <0.0001 | Yes |
| Types of particles | 3 | 10340 | 3447 | 2852 | <0.0001 | Yes |
| Concentration | 4 | 1571 | 392.7 | 374.5 | <0.0001 | yes |
| Residual | 40 | 205.3 | 5.133 |
| Source of Variation | Df | Sum-of-Squares | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Interaction | 6 | 1339 | 223.2 | 21.02 | <0.0001 | Yes |
| Types of particles | 3 | 27620 | 9206 | 866.8 | <0.0001 | Yes |
| Concentration | 2 | 3197 | 1599 | 150.5 | <0.0001 | Yes |
| Residual | 24 | 254.9 | 10.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilshad, E.; Bibi, M.; Sheikh, N.A.; Tamrin, K.F.; Mansoor, Q.; Maqbool, Q.; Nawaz, M. Synthesis of Functional Silver Nanoparticles and Microparticles with Modifiers and Evaluation of Their Antimicrobial, Anticancer, and Antioxidant Activity. J. Funct. Biomater. 2020, 11, 76. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11040076
Dilshad E, Bibi M, Sheikh NA, Tamrin KF, Mansoor Q, Maqbool Q, Nawaz M. Synthesis of Functional Silver Nanoparticles and Microparticles with Modifiers and Evaluation of Their Antimicrobial, Anticancer, and Antioxidant Activity. Journal of Functional Biomaterials. 2020; 11(4):76. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11040076
Chicago/Turabian StyleDilshad, Erum, Mehmoona Bibi, Nadeem Ahmed Sheikh, Khairul Fikri Tamrin, Qaisar Mansoor, Qaisar Maqbool, and Muhammad Nawaz. 2020. "Synthesis of Functional Silver Nanoparticles and Microparticles with Modifiers and Evaluation of Their Antimicrobial, Anticancer, and Antioxidant Activity" Journal of Functional Biomaterials 11, no. 4: 76. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11040076