Electrospun Poly(butylene-adipate-co-terephthalate)/Nano-hyDroxyapatite/Graphene Nanoribbon Scaffolds Improved the In Vivo Osteogenesis of the Neoformed Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Solutions
2.2. Production of Scaffolds
2.3. Characterization
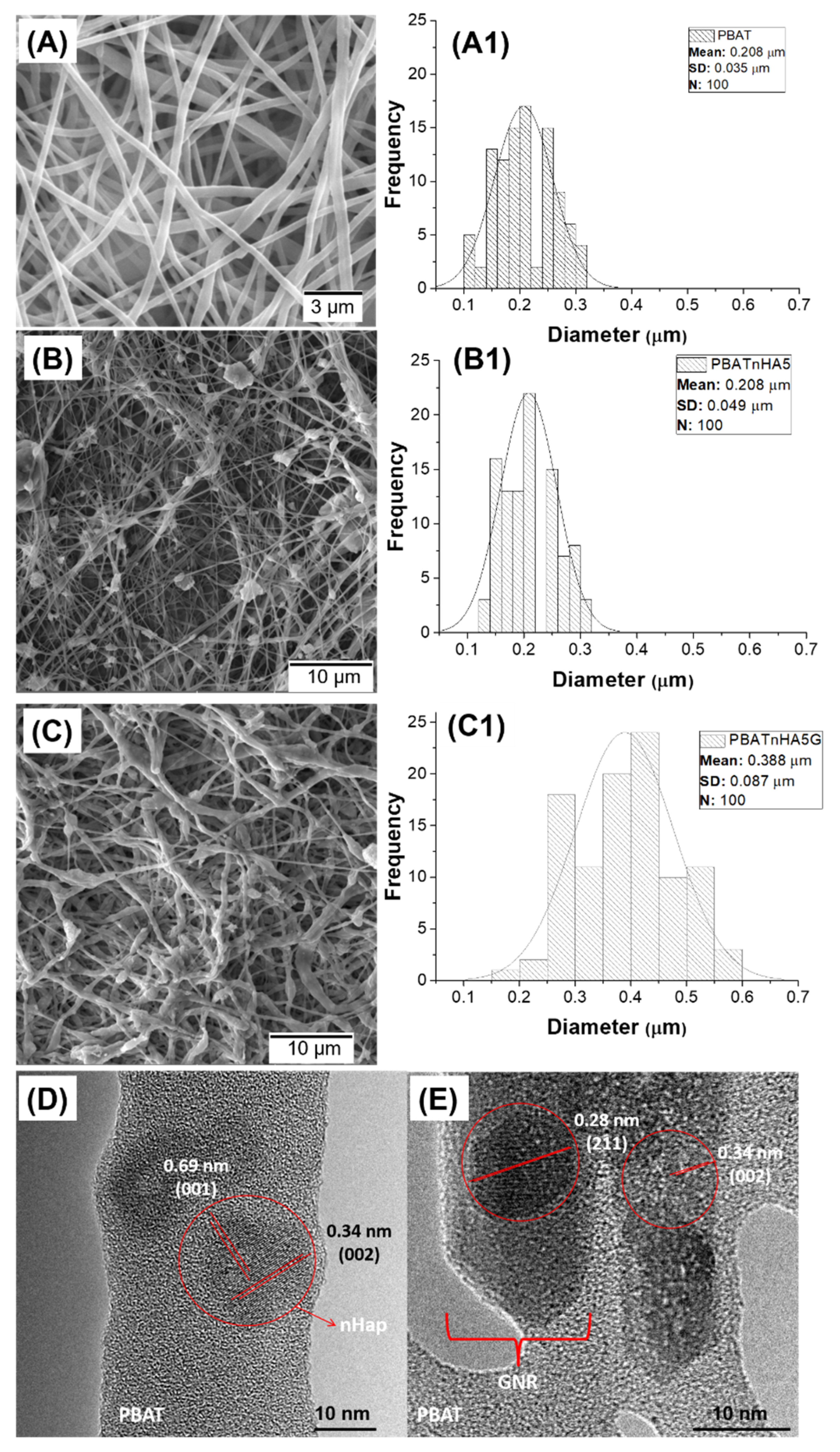
2.3.1. Morphological and Structural Analyses
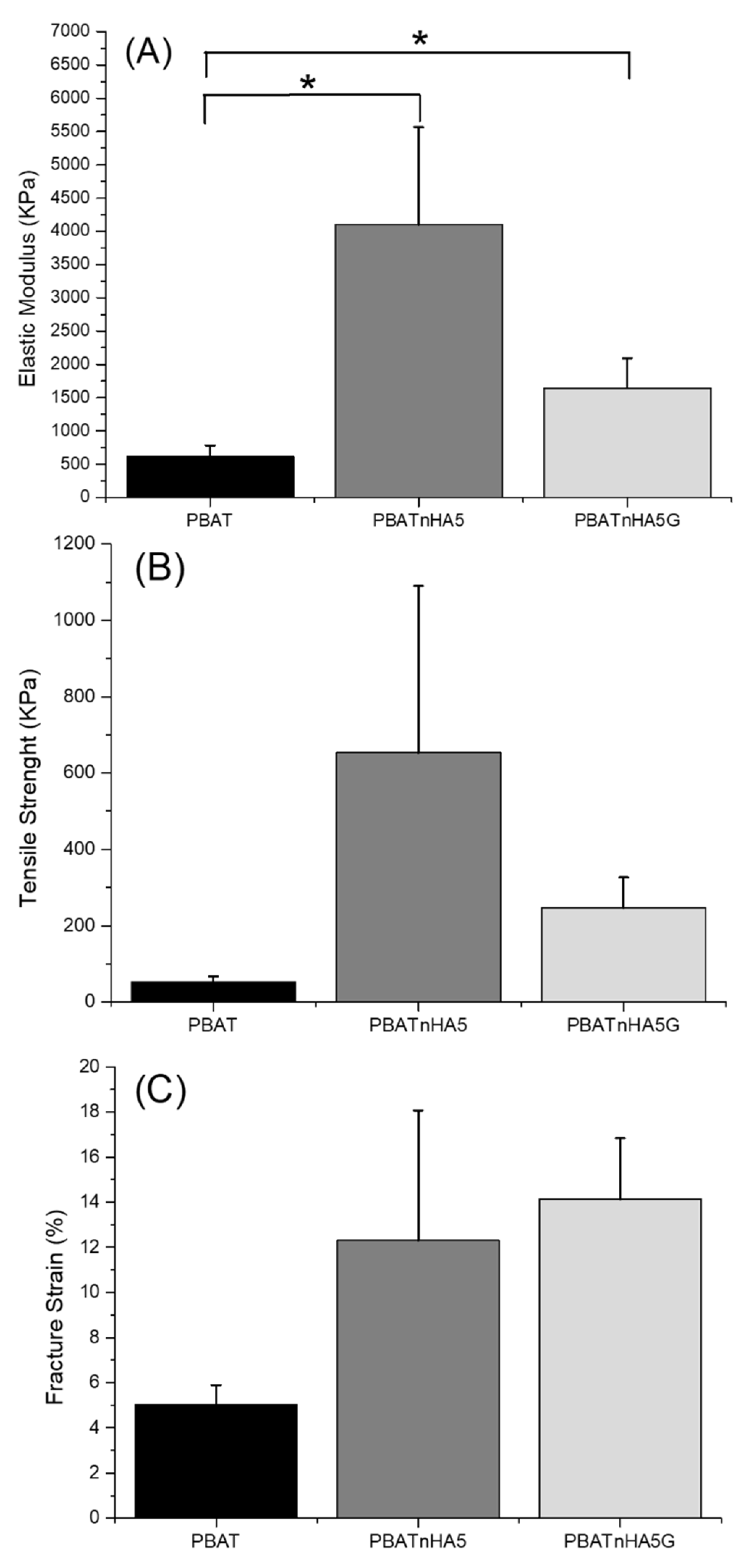
2.3.2. Mechanical Properties
2.4. In Vivo Analysis
2.4.1. Surgery Procedures
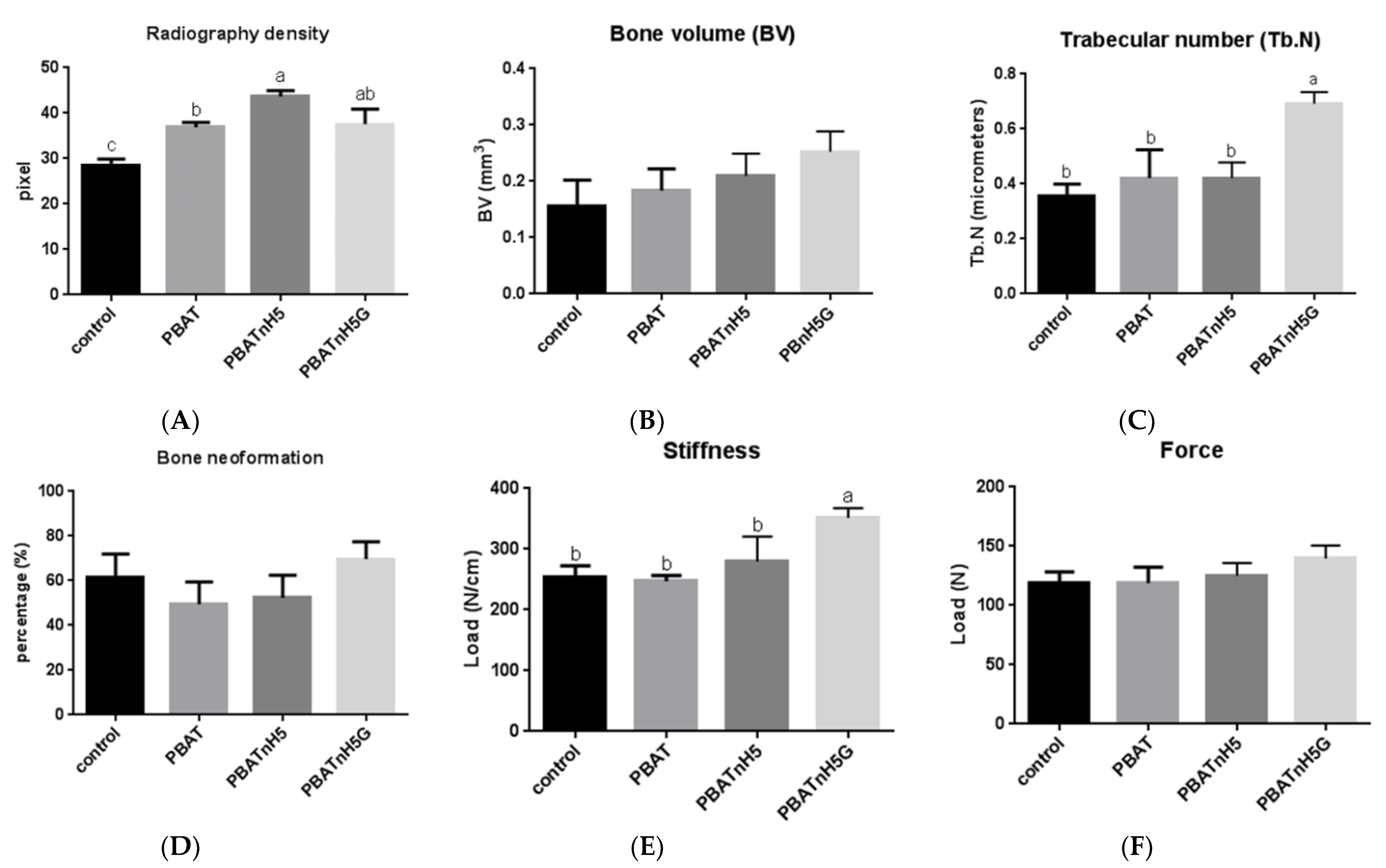
2.4.2. Radiography Analysis
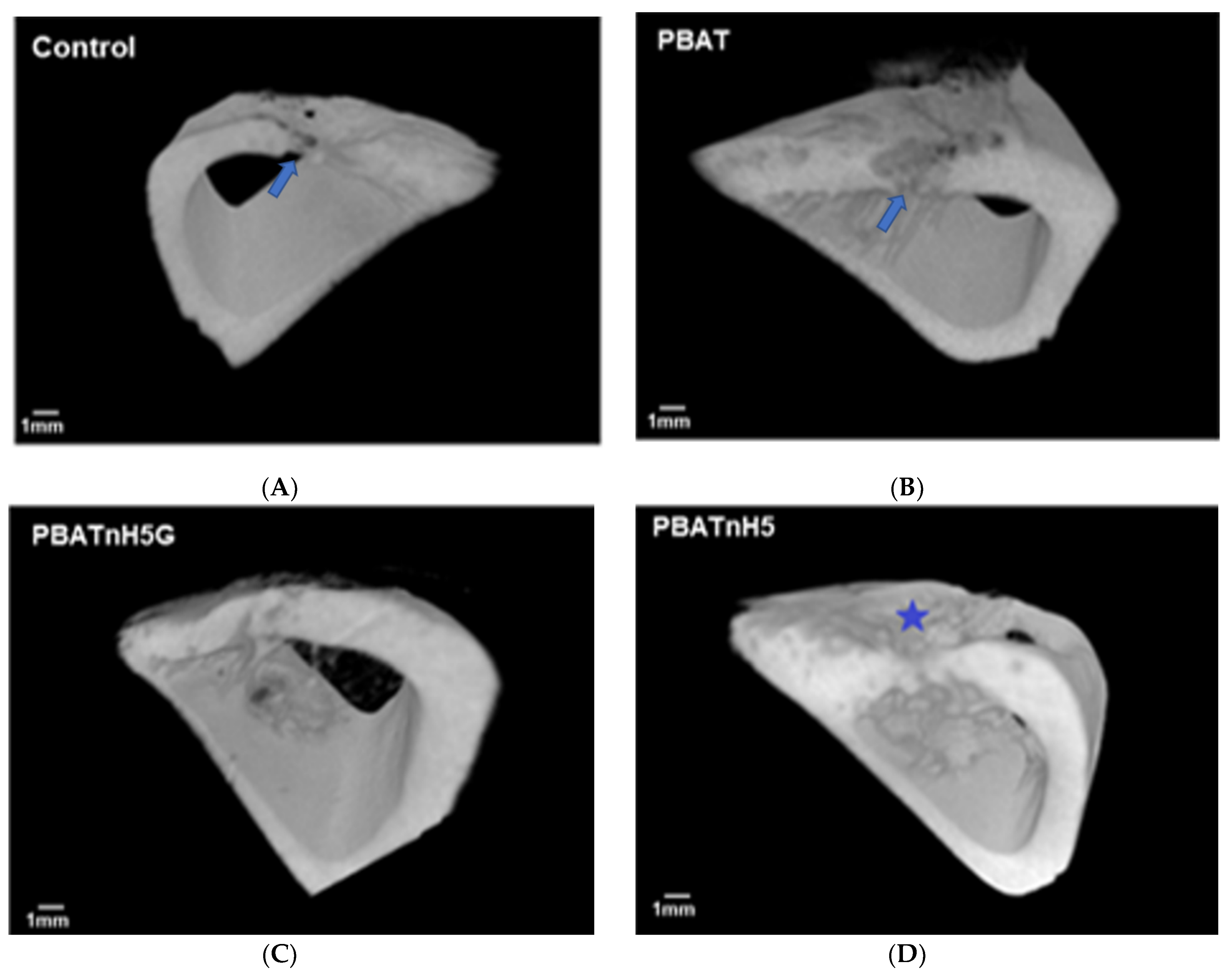
2.4.3. Microcomputed Tomography
2.4.4. Analysis of Bone Remodeling
2.4.5. Analysis of Biomechanical Properties
3. Results and Discussion
3.1. Characterization of Designed Scaffolds
3.2. Bone Repair Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berton, F.; Porrelli, D.; Di Lenarda, R.; Turco, G. A Critical Review on the Production of Electrospun Nanofibres for Guided Bone Regeneration in Oral Surgery. Nanomaterials 2019, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Chahal, S.; Kumar, A.; Hussian, F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering. A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 1308–1355. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 31701255. [Google Scholar] [CrossRef] [Green Version]
- Udomluck, N.; Koh, W.G.; Lim, D.J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, A.; Cakmak, S.; Cengiz, A.; Gumusderelioglu, M. Poly(butylene adipate-co-terephthalate) scaffolds: Processing, structural characteristics and cellular responses. J. Biomater. Sci. Polym. Ed. 2016, 27, 1841–1859. [Google Scholar] [CrossRef]
- Arslan, A.; Cakmak, S.; Gumusderelioglu, M. Enhanced osteogenic activity with boron-doped nanohydroxyapatite-loaded poly (butylene adipate-co-terephthalate) fibrous 3D matrix. Artif. Cells Nanomed. Biotechnol. 2018, 46, 790–799. [Google Scholar] [CrossRef] [Green Version]
- de Castro, J.G.; Rodrigues, B.V.M.; Ricci, R.; Costa, M.M.; Ribeiro, A.F.C.; Marciano, F.R.; Lobo, A.O. Designing a novel nanocomposite for bone tissue engineering using electrospun conductive PBAT/polypyrrole as a scaffold to direct nanohydroxyapatite electrodeposition. RSC Adv. 2016, 6, 32615–32623. [Google Scholar] [CrossRef]
- Elias, C.D.V.; Maia, A.L.M.; da Silva, L.R.; do Amaral, F.P.D.; Webster, T.J.; Marciano, F.R.; Lobo, A.O. In Vivo Evaluation of the Genotoxic Effects of Poly(Butylene adipate-co-terephthalate)/Polypyrrole with Nanohydroxyapatite Scaffolds for Bone Regeneration. Materials 2019, 12, 1330. [Google Scholar] [CrossRef] [Green Version]
- Neto, W.; Santos, J.; Averous, L.; Schlatter, G.; Bretas, R. Composites Structures for Bone Tissue Reconstruction. In Proceedings of the Preface: 30th International Conference of the Polymer, Cleveland, OH, USA, 6–12 June 2014; AIP Publishing: Melville, NY, USA, 2015. [Google Scholar]
- Neto, W.A.R.; de Paula, A.C.C.; Martins, T.M.M.; Goes, A.M.; Averous, L.; Schlatter, G.; Bretas, R.E.S. Poly(butylene adipate-co-terephthalate)/hydroxyapatite composite structures for bone tissue recovery. Polym. Degrad. Stab. 2015, 120, 61–69. [Google Scholar] [CrossRef]
- Rodrigues, B.V.M.; Silva, A.S.; Melo, G.F.S.; Vasconscellos, L.M.R.; Marciano, F.R.; Lobo, A.O. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly(butylene adipate-co-terephthalate) fibers. Mat. Sci. Eng. C Mater. 2016, 59, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Santana-Melo, G.F.; Rodrigues, B.V.M.; da Silva, E.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Vasconcellos, L.M.R.; Lobo, A.O. Electrospun ultrathin PBAT/nHAp fibers influenced the in vitro and in vivo osteogenesis and improved the mechanical properties of neoformed bone. Colloids Surf. B 2017, 155, 544–552. [Google Scholar] [CrossRef]
- Silva, A.D.; Rodrigues, B.V.M.; Oliveira, F.C.; Carvalho, J.O.; de Vasconcellos, L.M.R.; de Araujo, J.C.R.; Marciano, F.R.; Lobo, A.O. Characterization and in vitro and in vivo assessment of poly(butylene adipate-co-terephthalate)/nano-hydroxyapatite composites as scaffolds for bone tissue engineering. J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M. An overview on properties and applications of poly (butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Siqueira, I.A.; Corat, M.A.F.; Cavalcanti, B.d.N.; Neto, W.A.R.; Martin, A.A.; Bretas, R.E.S.; Marciano, F.R.; Lobo, A.O. In vitro and in vivo studies of novel poly(D, L-lactic acid), superhydrophilic carbon nanotubes, and nanohydroxyapatite scaffolds for bone regeneration. ACS Appl. Mater. Interfaces 2015, 7, 9385–9398. [Google Scholar] [CrossRef]
- Zanin, H.; Rosa, C.; Eliaz, N.; May, P.; Marciano, F.; Lobo, A. Assisted deposition of nano-hydroxyapatite onto exfoliated carbon nanotube oxide scaffolds. Nanoscale 2015, 7, 10218–10232. [Google Scholar] [CrossRef]
- Grinet, M.A.; Zanin, H.; Granato, A.E.C.; Porcionatto, M.; Marciano, F.R.; Lobo, A.O. Fast preparation of free-standing nanohydroxyapatite–vertically aligned carbon nanotube scaffolds. J. Mater. Chem. B 2014, 2, 1196–1204. [Google Scholar] [CrossRef]
- Rodrigues, B.V.; Leite, N.C.; das Neves Cavalcanti, B.; da Silva, N.S.; Marciano, F.R.; Corat, E.J.; Webster, T.J.; Lobo, A.O. Graphene oxide/multi-walled carbon nanotubes as nanofeatured scaffolds for the assisted deposition of nanohydroxyapatite: Characterization and biological evaluation. Int. J. Nanomed. 2016, 11, 2569. [Google Scholar]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Yuan, J.; Si, Y.; Chen, H. Large-scale molecular simulations on the mechanical response and failure behavior of a defective graphene: Cases of 5-8-5 defects. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wang, S.; Guo, Y.; Yuan, J.; Si, Y.; Zhang, S.; Chen, H. Strength and failure behavior of a graphene sheet containing bi-grain-boundaries. RSC Adv. 2014, 4, 54677–54683. [Google Scholar] [CrossRef]
- Andrade, T.M.; Mello, D.C.R.; Elias, C.M.V.; Abdala, J.M.A.; Silva, E.; Vasconcellos, L.M.R.; Tim, C.R.; Marciano, F.R.; Lobo, A.O. In vitro and in vivo evaluation of rotary-jet-spun poly(-caprolactone) with high loading of nano-hydroxyapatite. J. Mater. Sci. Mater. Med. 2019, 30, 30689050. [Google Scholar] [CrossRef]
- Hollanda, L.M.; Lobo, A.O.; Lancellotti, M.; Berni, E.; Corat, E.J.; Zanin, H. Graphene and carbon nanotube nanocomposite for gene transfection. Mater. Sci. Eng. C 2014, 39, 288–298. [Google Scholar] [CrossRef]
- Ricci, R.; Leite, N.C.S.; da-Silva, N.S.; Pacheco-Soares, C.; Canevari, R.A.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Graphene oxide nanoribbons as nanomaterial for bone regeneration: Effects on cytotoxicity, gene expression and bactericidal effect. Mater. Sci. Eng. C 2017, 78, 341–348. [Google Scholar] [CrossRef]
- Medeiros, J.S.; Oliveira, A.M.; Carvalho, J.O.D.; Ricci, R.; Martins, M.D.C.C.; Rodrigues, B.V.M.; Webster, T.J.; Viana, B.C.; Vasconcellos, L.M.R.; Canevari, R.A.; et al. Nanohydroxyapatite/Graphene Nanoribbons Nanocomposites Induce in Vitro Osteogenesis and Promote in Vivo Bone Neoformation. ACS Biomater. Sci. Eng. 2018, 4, 1580–1590. [Google Scholar] [CrossRef]
- de Vasconcellos, L.M.R.; do Prado, R.F.; Sartori, E.M.; Mendonça, D.B.S.; Mendonça, G.; Marciano, F.R.; Lobo, A.O. In vitro osteogenesis process induced by hybrid nanohydroxyapatite/graphene nanoribbons composites. J. Mater. Sci. Mater. Med. 2019, 30, 81. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.C.; Carvalho, J.O.; Gusmão, S.B.S.; Gonçalves, L.d.S.; Soares Mendes, L.M.; Freitas, S.A.P.; Gusmão, G.O.d.M.; Viana, B.C.; Marciano, F.R.; Lobo, A.O. High loads of nano-hydroxyapatite/graphene nanoribbon composites guided bone regeneration using an osteoporotic animal model. Int. J. Nanomed. 2019, 14, 865–874. [Google Scholar] [CrossRef] [Green Version]
- NIH IMAGE. Available online: https://imagej.nih.gov/nih-image/ (accessed on 4 February 2021).
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cerebr. Blood. Flow Met. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- de Vasconcellos, L.M.R.; Barbara, M.A.M.; da Silva Rovai, E.; de Oliveira França, M.; Ebrahim, Z.F.; de Vasconcellos, L.G.O.; Porto, C.D.; Cairo, C.A.A. Titanium scaffold osteogenesis in healthy and osteoporotic rats is improved by the use of low-level laser therapy (GaAlAs). Laser Med. Sci. 2016, 31, 899–905. [Google Scholar] [CrossRef] [Green Version]
- de Vasconcellos, L.M.R.; Barbara, M.A.M.; Deco, C.P.; Junqueira, J.C.; do Prado, R.F.; Anbinder, A.L.; de Vasconcellos, L.G.O.; Cairo, C.A.A.; Carvalho, Y.R. Healing of normal and osteopenic bone with titanium implant and low-level laser therapy (GaAlAs): A histomorphometric study in rats. Laser Med. Sci. 2014, 29, 575–580. [Google Scholar] [CrossRef]
- Vasconcellos, L.M.R.; Elias, C.d.M.V.; Minhoto, G.B.; Abdala, J.M.A.; Andrade, T.M.; de Araujo, J.C.R.; Gusmão, S.B.S.; Viana, B.C.; Marciano, F.R.; Lobo, A.O. Rotary-jet spun polycaprolactone/nano-hydroxyapatite scaffolds modified by simulated body fluid influenced the flexural mode of the neoformed bone. J. Mater. Sci. Mater. Med. 2020, 31, 72. [Google Scholar] [CrossRef]
- Vasconcellos, L.M.R.; Oliveira, M.V.; Graça, M.L.A.; Vasconcellos, L.G.O.; Cairo, C.A.A.; Carvalho, Y.R. Design of dental implants, influence on the osteogenesis and fixation. J. Mater. Sci. Mater. Med. 2008, 19, 2851–2857. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Z.; Xie, K.Y.; Lu, W.Y.; Roohani-Esfahani, S.; Kondyurin, A.; Zreiqat, H. A facile method to in situ formation of hydroxyapatite single crystal architecture for enhanced osteoblast adhesion. J. Mater. Chem. 2012, 22, 19081–19087. [Google Scholar] [CrossRef]
- Lee, H.-B.; Hsu, H.-C.; Wu, S.-C.; Hsu, S.-K.; Wang, P.-H.; Ho, W.-F. Microstructure and characteristics of calcium phosphate layers on bioactive oxide surfaces of air-sintered titanium foams after immersion in simulated body fluid. Materials 2016, 9, 956. [Google Scholar] [CrossRef] [Green Version]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the Influence of Solubility and Dielectric Constant on Electrospinning Polycaprolactone Solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Meng, Z.X.; Zheng, W.; Li, L.; Zheng, Y.F. Fabrication and characterization of three-dimensional nanofiber membrance of PCL–MWCNTs by electrospinning. Mat. Sci. Eng. C 2010, 30, 1014–1021. [Google Scholar] [CrossRef]
- Saligheh, O.; Forouharshad, M.; Arasteh, R.; Eslami-Farsani, R.; Khajavi, R.; Yadollah Roudbari, B. The effect of multi-walled carbon nanotubes on morphology, crystallinity and mechanical properties of PBT/MWCNT composite nanofibers. J. Polym. Res. 2013, 20, 65. [Google Scholar] [CrossRef]
- Singh, A.K.; Rastogi, A.; Singh, V. Biomechanical comparison of dynamic condylar screw and locking compression plate fixation in unstable distal femoral fractures: An in vitro study. Indian J. Orthop. 2013, 47, 615. [Google Scholar] [CrossRef] [PubMed]
- Ueno, F.H.; Pisani, M.J.; Machado, A.N.; Rodrigues, F.L.; Fujiki, E.N.; Rodrigues, L.M.R. Estudo biomecânico da fixação da fratura sacroilíaca com barras de titânio e parafusos pediculares. Acta Ortop. Bras. 2015, 23, 154–157. [Google Scholar] [CrossRef] [Green Version]
- Baji, A.; Mai, Y.-W.; Abtahi, M.; Wong, S.-C.; Liu, Y.; Li, Q. Microstructure development in electrospun carbon nanotube reinforced polyvinylidene fluoride fibers and its influence on tensile strength and dielectric permittivity. Compos. Sci. Technol. 2013, 88, 1–8. [Google Scholar] [CrossRef]
- Stein, R.S.; Silva, J.B.; Silva, V.D.d. Comparative Study of Bone Neoformation Using Autologous Grafing and Three Replancements: Bone Defects in Rats. Rev. Bras. Ortop. 2009, 44, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Fernández, R.F.; Bucchi, C.; Navarro, P.; Beltrán, V.; Borie, E. Bone grafts utilized in dentistry: An analysis of patients’ preferences. BMC. Med. Ethics. 2015, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Maji, K. Biomaterials for Bone Tissue Engineering: Recent Advances and Challenges. In Orthopedic Biomaterials: Progress in Biology, Manufacturing, and Industry Perspectives; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 429–452. [Google Scholar]
- Afewerki, S.; Bassous, N.; Harb, S.; Palo-Nieto, C.; Ruiz-Esparza, G.U.; Marciano, F.R.; Webster, T.; Lobo, A.O. Advances in Antimicrobial and Osteoinductive Biomaterials. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–34. [Google Scholar]
- Dimitry, O.I.; Abdeen, Z.I.; Ismail, E.; Saad, A. Preparation and properties of elastomeric polyurethane/organically modified montmorillonite nanocomposites. J. Polym. Res. 2010, 17, 801–813. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chen, C.-C.; Yang, M.-C. Characterization of nanocomposites of poly(butylene adipate-co-terephthalate) blending with organoclay. J. Polym. Res. 2011, 18, 2151–2159. [Google Scholar] [CrossRef]
- Ahmadpoor, P.; Nateri, A.S.; Motaghitalab, V. The optical properties of PVA/TiO2 composite nanofibers. J. Appl. Polym. Sci. 2013, 130, 78–85. [Google Scholar] [CrossRef]
- Karimi, Z.; Seyedjafari, E.; Mahdavi, F.S.; Hashemi, S.M.; Khojasteh, A.; Kazemi, B.; Mohammadi-Yeganeh, S. Baghdadite nanoparticle-coated poly l-lactic acid (PLLA) ceramics scaffold improved osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. J. Biomed. Mate. Res. A 2019, 107, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Wu, M.-H.; Bocchini, S.; Rasyida, A.; Yang, M.-C. PBAT based nanocomposites for medical and industrial applications. Mater. Science. Eng. C 2012, 32, 1331–1351. [Google Scholar] [CrossRef]
- Ribeiro, J.C.V.; Vieira, R.S.; Melo, I.M.; Araújo, V.M.A.; Lima, V. Versatility of chitosan-based biomaterials and their use as scaffolds for tissue regeneration. Sci. World J. 2017, 2017, 8639898. [Google Scholar] [CrossRef] [Green Version]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomaterials 2019, 9, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Ishikawa, K. Effects of nanopores on the mechanical strength, osteoclastogenesis, and osteogenesis in honeycomb scaffolds. J. Mater. Chem. B 2020, 8, 8536–8545. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]

| Groups | Named | nHAp (%) | GNR (%) |
|---|---|---|---|
| PBAT | PBAT | - | - |
| PBAT/nHAp | PBATnH5 | 5 | - |
| PBAT/nHAp/GNR | PBATnH5G | 5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcellos, L.M.R.; Santana-Melo, G.F.; Silva, E.; Pereira, V.F.; Araújo, J.C.R.; Silva, A.D.R.; Furtado, A.S.A.; Elias, C.d.M.V.; Viana, B.C.; Marciano, F.R.; et al. Electrospun Poly(butylene-adipate-co-terephthalate)/Nano-hyDroxyapatite/Graphene Nanoribbon Scaffolds Improved the In Vivo Osteogenesis of the Neoformed Bone. J. Funct. Biomater. 2021, 12, 11. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12010011
Vasconcellos LMR, Santana-Melo GF, Silva E, Pereira VF, Araújo JCR, Silva ADR, Furtado ASA, Elias CdMV, Viana BC, Marciano FR, et al. Electrospun Poly(butylene-adipate-co-terephthalate)/Nano-hyDroxyapatite/Graphene Nanoribbon Scaffolds Improved the In Vivo Osteogenesis of the Neoformed Bone. Journal of Functional Biomaterials. 2021; 12(1):11. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12010011
Chicago/Turabian StyleVasconcellos, Luana Marotta Reis, Gabriela F. Santana-Melo, Edmundo Silva, Vanessa Fernandes Pereira, Juliani Caroline Ribeiro Araújo, André Diniz Rosa Silva, André S. A. Furtado, Conceição de Maria Vaz Elias, Bartolomeu Cruz Viana, Fernanda Roberta Marciano, and et al. 2021. "Electrospun Poly(butylene-adipate-co-terephthalate)/Nano-hyDroxyapatite/Graphene Nanoribbon Scaffolds Improved the In Vivo Osteogenesis of the Neoformed Bone" Journal of Functional Biomaterials 12, no. 1: 11. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12010011






