Drastic Reduction of Bacterial, Fungal and Viral Pathogen Titers by Cuprous Oxide Impregnated Medical Textiles
Abstract
:1. Introduction
2. Materials and Methods
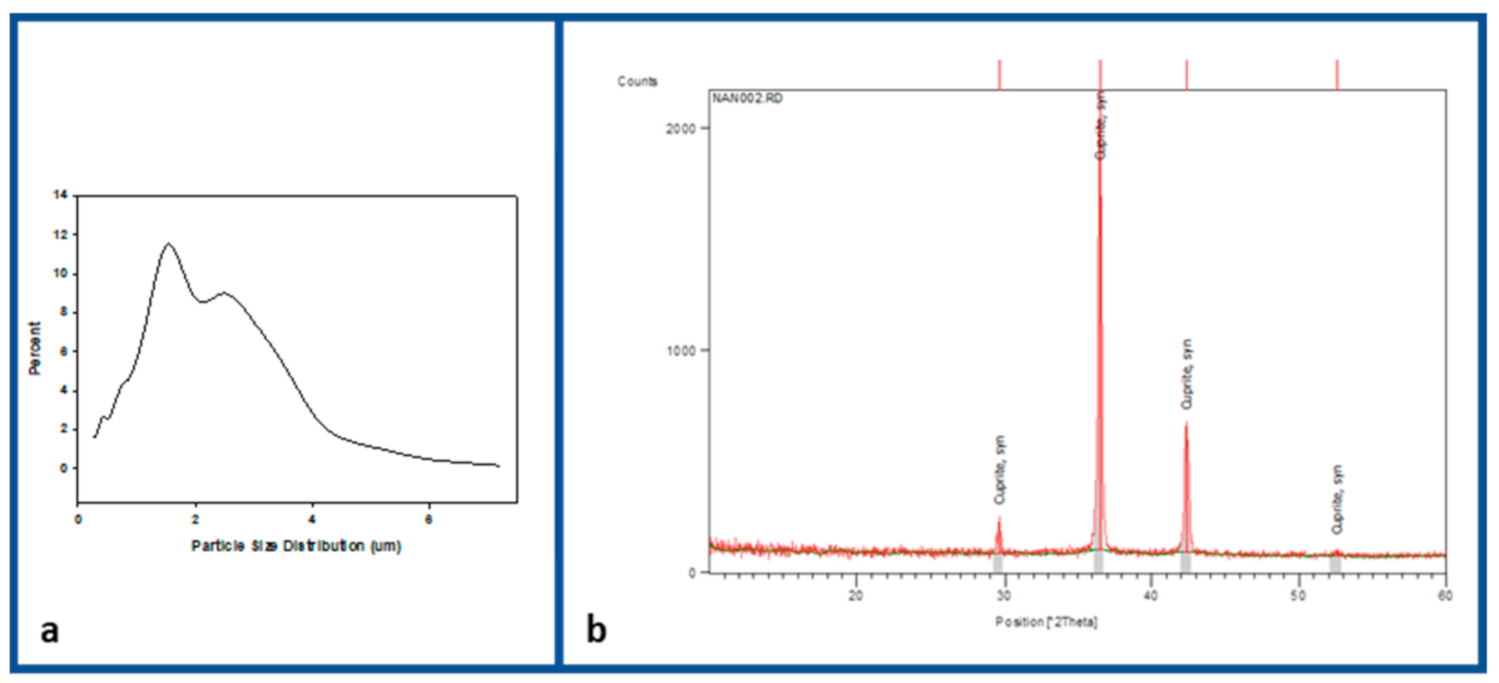
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehraeen, E.; Salehi, M.A.; Behnezhad, F.; Moghaddam, H.R.; Seyed Alinaghi, S. Transmission Modes of COVID-19: A Systematic Review. Infect. Disord. Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Ni, W.; Wang, Z.; Ma, G.; Pan, B.; Dong, L.; Gao, R.; Jiang, F. The distribution of SARS-CoV-2 contamination on the environmental surfaces during incubation period of COVID-19 patients. Ecotoxicol. Environ. Saf. 2020, 208, 111438. [Google Scholar] [CrossRef] [PubMed]
- Elsami, H.; Jalili, M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19). AMB Express 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Firquet, S.; Beaujard, S.; Lobert, P.E.; Sane, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140. [Google Scholar] [CrossRef] [Green Version]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246. [Google Scholar] [CrossRef] [Green Version]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235. [Google Scholar] [CrossRef] [Green Version]
- Borkow, G. Using copper to fight microorganisms. Curr. Chem. Biol. 2012, 6, 93. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.L.M.; Ducker, W.A. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723. [Google Scholar] [CrossRef]
- Doremalen, N.V.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Hans, M.; Hein, C.; Mancinelli, R.L.; Mucklich, F.; Wirth, R.; Rettberg, P.; Hellweg, C.E.; Moeller, R. Pure and Oxidized Copper Materials as Potential Antimicrobial Surfaces for Spaceflight Activities. Astrobiology 2017, 17, 1183. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mucklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, S.; Saphier, O.; Popov, M.; Shenker, M.; Entus, S.; Shotland, Y.; Saphier, M. Factors Enhancing the Antibacterial Effect of Monovalent Copper Ions. Curr. Microbiol. 2020, 77, 361. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of polycrystalline copper thin films at ambient conditions. J. Phys. Chem. C 2008, 112, 1101. [Google Scholar] [CrossRef]
- Weissenrieder, J.; Schreiner, M.; Leygraf, C. Comparison of the early stages of corrosion of copper and iron investigated by in situ TM-AFM. Appl. Surf. Sci. 2002, 193, 245. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper-impregnated products with potent biocidal activities. FASEB J. 2004, 18, 1728. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Endowing textiles with permanent potent biocidal properties by impregnating them with copper oxide. JTATM 2006, 5, 1. [Google Scholar]
- Burke, G.H.; Butler, J.P. Analysis of the role of copper impregnated composite hard surfaces, bed linens and patient gowns in reducing healthcare-associated infection rates. Int. J. Infect. Control 2018, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Sifri, C.D.; Burke, G.H.; Enfield, K.B. Reduced health care-associated infections in an acute care community hospital using a combination of self-disinfecting copper-impregnated composite hard surfaces and linens. Am. J. Infect. Control 2016, 44, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, J.P. Effect of copper-impregnated composite bed linens and patient gowns on healthcare-associated infection rates in six hospitals. J. Hosp. Infect. 2018, 100, e130–e134. [Google Scholar] [CrossRef] [PubMed]
- Lazary, A.; Weinberg, I.; Vatine, J.J.; Jefidoff, A.; Bardenstein, R.; Borkow, G.; Ohana, N. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int. J. Infect. Dis. 2014, 24, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, E.L.; Yosef, H.; Borkow, G.; Caine, Y.; Sasson, A.; Moses, A.E. Reduction of health care-associated infection indicators by copper oxide-impregnated textiles: Crossover, double-blind controlled study in chronic ventilator-dependent patients. Am. J. Infect. Control 2017, 45, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbay, J.; Mishal, J.; Magen, E.; Zatcoff, R.C.; Shemer-Avni, Y.; Borkow, G. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006, 35, 323. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, Y.H.; Leung, N.H.L.; Cowling, B.J.; Yang, Z.F. Role of viral bioaerosols in nosocomial infections and measures for prevention and control. J. Aerosol Sci. 2018, 117, 200. [Google Scholar] [CrossRef]
- Heinzerling, A.; Stuckey, M.J.; Scheuer, T.; Xu, K.; Perkins, K.M.; Resseger, H.; Magill, S.; Verani, J.R.; Jain, S.; Acosta, M.; et al. Transmission of COVID-19 to Health Care Personnel During Exposures to a Hospitalized Patient—Solano County, California. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 472. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990. [Google Scholar] [CrossRef]
- Creamer, E.; Humphreys, H. The contribution of beds to healthcare-associated infection: The importance of adequate decontamination. J. Hosp. Infect. 2008, 69, 8. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K. The role of textiles as fomites in the healthcare environment: A review of the infection control risk. PeerJ 2020, 8, e9790. [Google Scholar] [CrossRef] [PubMed]
- Gerhardts, A.; Hammer, T.R.; Balluff, C.; Mucha, H.; Hoefer, D. A model of the transmission of micro-organisms in a public setting and its correlation to pathogen infection risks. J. Appl. Microbiol. 2012, 112, 614. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lazaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A. Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 2012, 36, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds Journal of Hazardous Materials. J. Hazard. Mater. 2016, 312, 1. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borkow, G.; Sidwell, R.W.; Smee, D.F.; Barnard, D.L.; Morrey, J.D.; Lara-Villegas, H.H.; Shemer-Avni, Y.; Gabbay, J. Neutralizing viruses in suspensions by copper oxide based filters. Antimicrob. Agents Chemother. 2007, 51, 2605. [Google Scholar] [CrossRef] [Green Version]
- Borkow, G.; Lara, H.H.; Covington, C.Y.; Nyamathi, A.; Gabbay, J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 2008, 52, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borkow, G.; Okon-Levy, N.; Gabbay, J. Copper oxide impregnated wound dressings: Biocidal and safety studies. Wounds 2010, 22, 301–310. [Google Scholar]
- Weinberg, I.; Lazary, A.; Jefidoff, A.; Vatine, J.-J.; Borkow, G.; Ohana, N. Safety of using diapers containing copper oxide in chronic care elderly patients. Open Biol. J. 2013, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Borkow, G. Safety of using copper oxide in medical devices and consumer products. Curr. Chem. Biol. 2012, 6, 86. [Google Scholar] [CrossRef]

| Bacteria | Sample | Exposure | Percent | Log |
|---|---|---|---|---|
| Time (h) | Reduction | Reduction | ||
| MRSA | Test Sample 1 | 2 | >99.99 | >4 |
| Test Sample 2 | 2 | >99.99 | >4 | |
| Control Fabric | 2 | 0 | 0 | |
| K. pneumoniae | Test Sample 1 | 2 | >99.98 | >3.9 |
| Test Sample 2 | 2 | >99.99 | >4 | |
| Control Fabric | 2 | 0 | 0 | |
| E. Fecalis | Test Sample 1 | 2 | >99.99 | >4 |
| Test Sample 2 | 2 | >99.99 | >4 | |
| Control Fabric | 2 | 0 | 0 | |
| C. difficile | Test Sample 1 | 2 | 97.83 | 1.66 |
| Test Sample 1 | 6 | >99.91 | >3 | |
| Test Sample 1 | 18 | >99.99 | >4 | |
| Test Sample 2 | 2 | 98.99 | 2.99 | |
| Test Sample 2 | 6 | >99.91 | >3 | |
| Test Sample 2 | 18 | >99.99 | >4 | |
| Control Fabric | 2/6/18 | 0 | 0 | |
| C. auris | Test Sample 1 | 2 | >99.99 | >4 |
| Test Sample 2 | 2 | >99.99 | >4 | |
| Control Fabric | 2 | 0 | 0 |
| Test Sample | Contact Time (h) | Staphylococcusaureus | Klebsiellapneumoniae | Enterobacteraerogenes |
|---|---|---|---|---|
| 1 | 0 | 6.25 × 105 | 2.70 × 105 | 5.65 × 105 |
| 24 | 3.00 × 102 | 1.00 × 102 | 1.00 × 102 | |
| % reduction | >99.9 | >99.9 | >99.9 | |
| 2 | 0 | 6.25 × 105 | 2.70 × 105 | 5.65 × 105 |
| 24 | 3.00 × 103 | 1.00 × 102 | 7.50 × 102 | |
| % reduction | 99.52 | >99.9 | 99.87 |
| Dilution | Initial | Incubation Time | Controls | |||
|---|---|---|---|---|---|---|
| Sample | Factor | Inoculum | 0 h | 2 h | Extraction | Neutralization |
| Control Sample | 10−1 | 6 | 6 | 6 | 0 | 0 |
| 10−2 | 6 | 6 | 6 | 0 | 0 | |
| 10−3 | 6 | 6 | 6 | 0 | 0 | |
| 10−4 | 4 | 5 | 2 | 0 | 0 | |
| 10−5 | 2 | 0 | 0 | 0 | 0 | |
| 10−6 | 0 | 0 | 0 | 0 | 0 | |
| 10−7 | 0 | 0 | 0 | 0 | 0 | |
| Test Sample 1 | 10−1 | 6 | 6 | 6 | 0 | 0 |
| 10−2 | 6 | 6 | 0 | 0 | 0 | |
| 10−3 | 6 | 6 | 0 | 0 | 0 | |
| 10−4 | 4 | 5 | 0 | 0 | 0 | |
| 10−5 | 2 | 0 | 0 | 0 | 0 | |
| 10−6 | 0 | 0 | 0 | 0 | 0 | |
| 10−7 | 0 | 0 | 0 | 0 | 0 | |
| Test Sample 2 | 10−1 | 6 | 6 | 6 | 0 | 0 |
| 10−2 | 6 | 6 | 0 | 0 | 0 | |
| 10−3 | 6 | 6 | 0 | 0 | 0 | |
| 10−4 | 4 | 5 | 0 | 0 | 0 | |
| 10−5 | 2 | 0 | 0 | 0 | 0 | |
| 10−6 | 0 | 0 | 0 | 0 | 0 | |
| 10−7 | 0 | 0 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkow, G.; Salvatori, R.; Kanmukhla, V.K. Drastic Reduction of Bacterial, Fungal and Viral Pathogen Titers by Cuprous Oxide Impregnated Medical Textiles. J. Funct. Biomater. 2021, 12, 9. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12010009
Borkow G, Salvatori R, Kanmukhla VK. Drastic Reduction of Bacterial, Fungal and Viral Pathogen Titers by Cuprous Oxide Impregnated Medical Textiles. Journal of Functional Biomaterials. 2021; 12(1):9. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12010009
Chicago/Turabian StyleBorkow, Gadi, Rachel Salvatori, and Vikram K. Kanmukhla. 2021. "Drastic Reduction of Bacterial, Fungal and Viral Pathogen Titers by Cuprous Oxide Impregnated Medical Textiles" Journal of Functional Biomaterials 12, no. 1: 9. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12010009




