Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Physicochemical Characterization
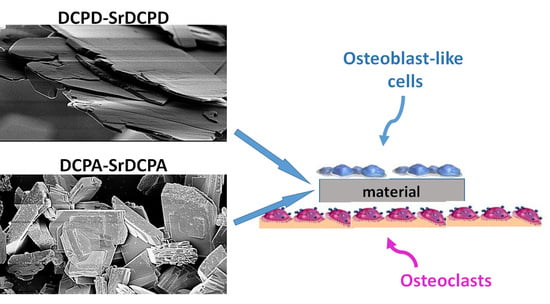
2.2. In Vitro Co-Culture Model
2.3. Cell Viability
2.4. Osteoclast Differentiation
2.5. Gene Expression
2.6. Immunoenzymatic Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Materials Synthesis and Characterization
3.2. Cellular Tests
3.2.1. Cell Viability and Morphology
3.2.2. Gene Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.; Chow, L.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Resorpt. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Nihouannen, D.L.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dehydrate bioceramics: Brushite vs. monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A. Dicalcium phosphate anhydrous: An appropriate bioceramic in regeneration of critical-sized radial bone defects in rats. Calcif. Tissue Int. 2017, 101, 530–544. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, C.C.; Xu, L.; Wang, Q. Preparation of dicalcium phosphate anhydrous (monetite) biological coating on titanium by spray-drying method. Adv. Mater. Sci. Eng. 2017, 2017, 8281523. [Google Scholar] [CrossRef] [Green Version]
- Klammert, U.; Reuther, T.; Jahn, C.; Kraski, B.; Kübler, A.C.; Gbureck, U. Cytocompatibility of brushite and monetite cell culture scaffolds made by three-dimensional powder printing. Acta Biomater. 2009, 5, 727–734. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; van Blitterswijk, C.A.; Barralet, J.E. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Rubini, K.; Boanini, E.; Bigi, A. Role of aspartic and polyaspartic acid on the synthesis and hydrolysis of brushite. J. Funct. Biomater. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catti, M.; Ferraris, G.; Filhol, A. Hydrogen bonding in the crystalline state. CaHPO4 (Monetite), P-1 or PI? A novel neutron diffraction study. Acta Crystallogr. 1977, 33, 1223–1229. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Gazzano, M. Ion substitution in biological and synthetic apatites. In Biomineralization and Biomaterials: Fundamentals and Applications; Aparicio, C., Ginebra, M.P., Eds.; Woodhead Publishing (Elsevier): Sawston, UK, 2016; pp. 235–266. [Google Scholar] [CrossRef]
- Laskus, A.; Kolmas, J. Ionic substitutions in non-apatitic calcium phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcos, D.; Vallet-Regi, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Felsenberg, D.; Brandi, M.L. How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporos. Int. 2011, 22, 1659–1667. [Google Scholar] [CrossRef]
- Tenti, S.; Cheleschi, S.; Guidelli, G.M.; Galeazzi, M.; Fioravanti, A. What about strontium ranelate in osteoarthritis? Doubts and securities. Mod. Rheumatol. 2014, 24, 881–884. [Google Scholar] [CrossRef]
- Schumacher, M.; Hens, A.; Rohnke, M.; Gelinki, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Contartese, D.; Bigi, A.; Boanini, E.; Parrilli, A.; Lolli, R.; Gasbarrini, A.; Barbanti Brodano, G.; Fini, M. Effect of strontium substituted ß-TCP associated to mesenchymal stem cells from bone marrow and adipose tissue on spinal fusion in healthy and ovariectomized rat. J. Cell. Physiol. 2019, 234, 20046–20056. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Parrilli, A.; Torricelli, P.; Boanini, E.; Bigi, A.; Fini, M. Antiresorptive properties of strontium substituted and alendronate functionalized hydroxyapatite nanocrystals in an ovariectomized rat spinal arthrodesis model. Mater. Sci. Eng. C 2019, 95, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Rubini, K.; Mazzeo, P.P.; Bigi, A. Structural interplay between strontium and calcium in α-CaHPO4 and β-SrHPO4. Ceram. Int. 2021, 47, 24412–24420. [Google Scholar] [CrossRef]
- Boudjada, A.; Masse, R.; Guttel, J.C. Structure cristalline de l’orthophosphate monoacide de strontium:SrHPO4 [alpha]: Forme triclinique. Acta Cryst. 1978, 34, 2692–2695. [Google Scholar] [CrossRef]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and hydrolysis of brushite (DCPD): The role of ionic substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- da Rocha, D.N.; de Oliveira Cruz, L.R.; de Campos, J.B.; dos Santos, J.L.; Blazutti Marçal, R.L.S.; Mijares, D.Q.; Barbosa, R.M.; Coelho, P.G.; da Silva, M.H.P. Bioactivity of strontium-monetite coatings for biomedical applications. Ceram. Int. 2019, 45, 7568–7579. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Fini, M.; Bigi, A. Antiresorptive and anti-angiogenetic octacalcium phosphate functionalized with bisphosphonates: An in vitro tri-culture study. Acta Biomater. 2017, 54, 419–428. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Mariana, B.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Idowu, B.; Cama, G.; Deb, S.; Di Silvio, L. In vitro osteoinductive potential of porous monetite for bone tissue engineering. J. Tissue Eng. 2014, 5, 2041731414536572. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, F.; Gao, W.; Chen, X.; Guo, Z.; Zhang, W. Strontium-substituted sub-micron bioactive glasses inhibit ostoclastogenesis through suppression of RANKL-induced signaling pathway. Regen. Biomater. 2020, 7, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Sample | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Sr Content (wt%) |
|---|---|---|---|---|---|---|---|
| DCPA | 6.891(8) | 6.639(5) | 6.996(4) | 96.15(5) | 103.96(1) | 88.51(1) | --- |
| SrDCPA | 6.927(9) | 6.649(8) | 7.014(5) | 96.08(8) | 104.07(1) | 88.56(2) | 5.8 |
| DCPD | 6.363(5) | 15.189(4) | 5.816(5) | --- | 118.58(1) | --- | --- |
| SrDCPD | 6.385(5) | 15.208(4) | 5.825(4) | --- | 118.56(1) | --- | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boanini, E.; Pagani, S.; Tschon, M.; Rubini, K.; Fini, M.; Bigi, A. Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization. J. Funct. Biomater. 2022, 13, 65. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb13020065
Boanini E, Pagani S, Tschon M, Rubini K, Fini M, Bigi A. Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization. Journal of Functional Biomaterials. 2022; 13(2):65. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb13020065
Chicago/Turabian StyleBoanini, Elisa, Stefania Pagani, Matilde Tschon, Katia Rubini, Milena Fini, and Adriana Bigi. 2022. "Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization" Journal of Functional Biomaterials 13, no. 2: 65. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb13020065