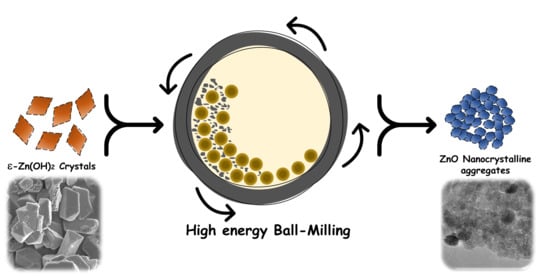
Solvent-Free Mechanochemical Synthesis of ZnO Nanoparticles by High-Energy Ball Milling of ε-Zn(OH)2 Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Characterization
3.1.1. XRD Analysis
3.1.2. Microscopy
3.2. Phase Transition Mechanism
3.2.1. TGA Analysis
3.2.2. XPS Analysis
3.3. Photocatalytic Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO Nanostructures for Dye-Sensitized Solar Cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Ahmad, M.; Yingying, S.; Nisar, A.; Sun, H.; Shen, W.; Wei, M.; Zhu, J. Synthesis of hierarchical flower-like ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high performance Li-ion battery anodes. J. Mater. Chem. 2011, 21, 7723–7729. [Google Scholar] [CrossRef]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.L. Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: A review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, J.; Xiang, Q.; Li, H.; Pan, Q.; Xu, P. Brush-Like Hierarchical ZnO Nanostructures: Synthesis, Photoluminescence and Gas Sensor Properties. J. Phys. Chem. C 2009, 113, 3430–3435. [Google Scholar] [CrossRef]
- Bamiduro, O.; Mustafa, H.; Mundle, R.; Konda, R.B.; Pradhan, A. Metal-like conductivity in transparent Al: ZnO films. Appl. Phys. Lett. 2007, 90, 252108. [Google Scholar] [CrossRef]
- Vinodkumar, R.; Navas, I.; Chalana, S.; Gopchandran, K.; Ganesan, V.; Philip, R.; Sudheer, S.; Pillai, V.P.M. Highly conductive and transparent laser ablated nanostructured Al: ZnO thin films. Appl. Surf. Sci. 2010, 257, 708–716. [Google Scholar] [CrossRef]
- King, D.M.; Liang, X.; Carney, C.S.; Hakim, L.F.; Li, P.; Weimer, A.W. Atomic Layer Deposition of UV-Absorbing ZnO Films on SiO2 and TiO2 Nanoparticles Using a Fluidized Bed Reactor. Adv. Funct. Mater. 2008, 18, 607–615. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Lee, J.S.; Lee, K.-H. Carbon-doped ZnO nanostructures synthesized using vitamin C for visible light photocatalysis. CrystEngComm 2010, 12, 3929–3935. [Google Scholar] [CrossRef] [Green Version]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K.; Babu, R.R.; Sethuraman, K.; Babu, S.M. Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 2017, 418, 138–146. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Ji, Y.; Ma, X.; Xu, A.J.; Que, D. Low Temperature Synthesis of Flowerlike ZnO Nanostructures by Cetyltrimethylammonium Bromide-Assisted Hydrothermal Process. J. Phys. Chem. B 2004, 108, 3955–3958. [Google Scholar] [CrossRef]
- Yang, L.-L.; Zhao, Q.; Willander, M.; Yang, J. Effective way to control the size of well-aligned ZnO nanorod arrays with two-step chemical bath deposition. J. Cryst. Growth 2009, 311, 1046–1050. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Cai, W. From ZnO Nanorods to Nanoplates: Chemical Bath Deposition Growth and Surface-Related Emissions. J. Phys. Chem. C 2008, 112, 680–685. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol-gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [Green Version]
- Zak, A.K.; Abrishami, M.E.; Majid, W.A.; Yousefi, R.; Hosseini, S.M. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol-gel combustion method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Demoisson, F.; Piolet, R.; Bernard, F. Hydrothermal synthesis of ZnO crystals from Zn (OH) 2 metastable phases at room to supercritical conditions. Cryst. Growth Des. 2014, 14, 5388–5396. [Google Scholar] [CrossRef]
- Moezzi, A.; Cortie, M.B.; McDonagh, A.M. Aqueous pathways for the formation of zinc oxide nanoparticles. Dalton Trans. 2011, 40, 4871–4878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xiang, L. Formation of ZnO rods with varying diameters from ε-Zn (OH) 2. J. Cryst. Growth 2014, 401, 279–284. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. The mechanism for hydrothermal growth of zinc oxide. CrystEngComm 2012, 14, 1232–1240. [Google Scholar] [CrossRef]
- Yan, X.; Chai, L.; Li, Q.; Ye, L.; Yang, B.; Wang, Q. Pathway of zinc oxide formation by seed-assisted and controlled double-jet precipitation. CrystEngComm 2016, 18, 924–929. [Google Scholar] [CrossRef]
- Wang, J.; Ma, P.; Xiang, L. Effects of NaOH on formation of ZnO nanorods from ε-Zn (OH) 2. Mater. Lett. 2015, 141, 118–121. [Google Scholar] [CrossRef]
- Sopicka-Lizer, M. High-Energy Ball Milling: Mechanochemical Processing of Nanopowders; woodhead publishing, 80 High Street Sawston Cambridge, CB22 3HJ United Kingdom; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Zhang, X.; Qiu, G. Mechanochemical synthesis of cobalt oxide nanoparticles. Mater. Lett. 2004, 58, 387–389. [Google Scholar] [CrossRef]
- Yuan, W.; O’Connor, J.; James, S.L. Mechanochemical synthesis of homo- and hetero-rare-earth (iii) metal-organic frameworks by ball milling. CrystEngComm 2010, 12, 3515–3517. [Google Scholar] [CrossRef]
- Brinks, H.W.; Istad-Lem, A.; Hauback, B.C. Mechanochemical Synthesis and Crystal Structure of α ‘-AlD3 and α-AlD3. J. Phys. Chem. B 2006, 110, 25833–25837. [Google Scholar] [CrossRef] [PubMed]
- Ravnsbæk, J.B.; Swager, T.M. Mechanochemical Synthesis of Poly (phenylene vinylenes). ACS Macro Lett. 2014, 3, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Giri, P.K.; Bhattacharyya, S.; Singh, D.K.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M. Correlation between microstructure and optical properties of ZnO nanoparticles synthesized by ball milling. J. Appl. Phys. 2007, 102, 093515. [Google Scholar] [CrossRef] [Green Version]
- Amirkhanlou, S.; Ketabchi, M.; Parvin, N. Nanocrystalline/nanoparticle ZnO synthesized by high energy ball milling process. Mater. Lett. 2012, 86, 122–124. [Google Scholar] [CrossRef]
- Glushenkov, A.M.; Zhang, H.; Chen, Y.I. Reactive ball milling to produce nanocrystalline ZnO. Mater. Lett. 2008, 62, 4047–4049. [Google Scholar] [CrossRef]
- Mahajan, B.K.; Yu, X.; Shou, W.; Pan, H.; Huang, X. Mechanically milled irregular zinc nanoparticles for printable bioresorbable electronics. Small 2017, 13, 1700065. [Google Scholar] [CrossRef]
- Ozcan, S.; Can, M.M.; Ceylan, A. Single step synthesis of nanocrystalline ZnO via wet-milling. Mater. Lett. 2010, 64, 2447–2449. [Google Scholar] [CrossRef]
- Piña-Pérez, Y.; Aguilar-Martínez, O.; Acevedo-Peña, P.; Santolalla-Vargas, C.; Oros-Ruíz, S.; Galindo-Hernández, F.; Gómez, R.; Tzompantzi, F. Novel ZnS-ZnO composite synthesized by the solvothermal method through the partial sulfidation of ZnO for H2 production without sacrificial agent. Appl. Catal. B Environ. 2018, 230, 125–134. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, L.; Kim, E.J.; Hahn, S.H. Electronic structure and optical properties of Zn (OH) 2: LDA + U calculations and intense yellow luminescence. RSC Adv. 2015, 5, 87496–87503. [Google Scholar] [CrossRef]
- Taylor, A.; Sinclair, H. On the determination of lattice parameters by the debye-scherrer method. Proc. Phys. Soc. 2002, 57, 126–135. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N.W. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 2002, 56, 978–982. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson—Hall and size—Strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Met. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Kibasomba, P.M.; Dhlamini, S.; Maaza, M.; Liu, C.-P.; Rashad, M.M.; Rayan, D.A.; Mwakikunga, B.W. Strain and grain size of TiO2 nanoparticles from TEM, Raman spectroscopy and XRD: The revisiting of the Williamson-Hall plot method. Results Phys. 2018, 9, 628–635. [Google Scholar] [CrossRef]
- Gonçalves, N.; Carvalho, J.; Lima, Z.; Sasaki, J. Size—strain study of NiO nanoparticles by X-ray powder diffraction line broadening. Mater. Lett. 2012, 72, 36–38. [Google Scholar] [CrossRef]
- Oleszak, D.; Shingu, P.H. Nanocrystalline metals prepared by low energy ball milling. J. Appl. Phys. 1996, 79, 2975–2980. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Paula, H.C.; de Paula, R.C. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Ghotbi, M.Y. Synthesis and characterization of nano-sized ɛ-Zn (OH) 2 and its decomposed product, nano-zinc oxide. J. Alloy. Compd. 2010, 491, 420–422. [Google Scholar] [CrossRef]
- Vohs, J.; Barteau, M. Dehydration and dehydrogenation of ethanol and 1-propanol on the polar surfaces of zinc oxide. Surf. Sci. 1989, 221, 590–608. [Google Scholar] [CrossRef]
- Nefedov, V.; Salyn, Y.; Leonhardt, G.; Scheibe, R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 121–124. [Google Scholar] [CrossRef]
- Valtiner, M.; Borodin, S.; Grundmeier, G. Preparation and characterisation of hydroxide stabilised ZnO (0001)-Zn-OH surfaces. Phys. Chem. Chem. Phys. 2007, 9, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Marrani, A.G.; Caprioli, F.; Boccia, A.; Zanoni, R.; Decker, F. Electrochemically deposited ZnO films: An XPS study on the evolution of their surface hydroxide and defect composition upon thermal annealing. J. Solid State Electrochem. 2013, 18, 505–513. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Weststrate, K.-J.; Dunnen, A.D.; Van Reijzen, M.E.; Hahn, C.; Juurlink, L.B.F. Hydrophilic Interaction Between Low-Coordinated Au and Water: H2O/Au (310) Studied with TPD and XPS. J. Phys. Chem. C 2016, 120, 8693–8703. [Google Scholar] [CrossRef]
- Martensson, N.; PA, M. Molecular and solid water, a comparative esca study. Nouv. J. Chim. 1977, 1, 191–196. [Google Scholar]
- Pargoletti, E.; Pifferi, V.; Falciola, L.; Facchinetti, G.; Depaolini, A.R.; Davoli, E.; Marelli, M.; Cappelletti, G. A detailed investigation of MnO2 nanorods to be grown onto activated carbon. High efficiency towards aqueous methyl orange adsorption/degradation. Appl. Surf. Sci. 2019, 472, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Dang, T.-D.; Banerjee, A.N.; Tran, Q.-T.; Roy, S. Fast degradation of dyes in water using manganese-oxide-coated diatomite for environmental remediation. J. Phys. Chem. Solids 2016, 98, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Jiang, R.; Fu, Y.; Guan, Y.; Yao, J.; Xiao, L.; Zeng, G. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 2012, 286, 41–48. [Google Scholar] [CrossRef]
- Mahendiran, M.; Mathen, J.; Racik, K.M.; Madhavan, J.; Raj, M.V.A. Facile synthesis of n-ZnO@ p-CuO nanocomposite for water purification enhanced decolorization of methyl orange. J. Mater. Sci. Mater. Electron. 2019, 30, 16099–16109. [Google Scholar] [CrossRef]
- Xie, J.; Wang, H.; Duan, M.; Zhang, L. Synthesis and photocatalysis properties of ZnO structures with different morphologies via hydrothermal method. Appl. Surf. Sci. 2011, 257, 6358–6363. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2014, 5, 3306–3351. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalination Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf. B Biointerfaces 2013, 103, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Cycles | Average FWHM (deg) | Crystallite Size (nm) | Strain (ε) | |
|---|---|---|---|---|
| Scherrer | Williamson-Hall | |||
| 2 | 0.54896 | 22.07 | 25.87 | 9.17 |
| 5 | 0.62866 | 17.57 | 23.82 | 1.68 |
| 8 | 0.87176 | 12.86 | 22.44 | 3.79 |
| 10 | 1.14171 | 9.51 | 13.45 | 3.33 |
| No of Cycles | Phase | Zn(OH)2 Percentage |
|---|---|---|
| 2 | ε-Zn(OH)2 + a-Zn(OH)2 | 25.9 |
| 5 | a-Zn(OH)2 | 34.1 |
| 8 | a-Zn(OH)2 | 12.7 |
| 10 | a-Zn(OH)2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otis, G.; Ejgenberg, M.; Mastai, Y. Solvent-Free Mechanochemical Synthesis of ZnO Nanoparticles by High-Energy Ball Milling of ε-Zn(OH)2 Crystals. Nanomaterials 2021, 11, 238. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11010238
Otis G, Ejgenberg M, Mastai Y. Solvent-Free Mechanochemical Synthesis of ZnO Nanoparticles by High-Energy Ball Milling of ε-Zn(OH)2 Crystals. Nanomaterials. 2021; 11(1):238. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11010238
Chicago/Turabian StyleOtis, Gil, Michal Ejgenberg, and Yitzhak Mastai. 2021. "Solvent-Free Mechanochemical Synthesis of ZnO Nanoparticles by High-Energy Ball Milling of ε-Zn(OH)2 Crystals" Nanomaterials 11, no. 1: 238. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11010238