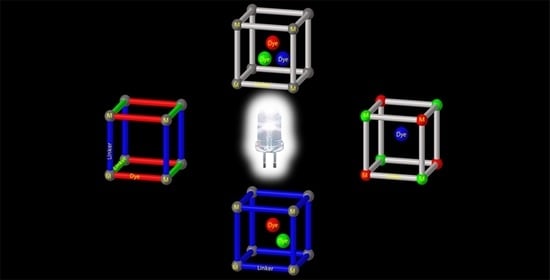
Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes
Abstract
:1. Introduction
2. Phosphors Excited by Blue-LED Chip
3. Phosphors Excited by UV-LED Chip
3.1. Luminescence from Organic Dyes
3.2. Luminescence from Dyes and Metals
3.3. Luminescence from Dyes and Organic Linkers
3.4. Organic Dyes as Fluorescent Linkers
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.Y.; Wang, X.L.; Zhang, X.; Qin, C.; Li, P.; Su, Z.M.; Zhu, D.X.; Shan, G.G.; Shao, K.Z.; Wu, H.; et al. Efficient and tunable white-light emission of metal-organic frameworks by iridium-complex encapsulation. Nat. Commun. 2013, 4, 2717–2724. [Google Scholar] [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lussem, B.; Leo, K. White organic light-emitting diodes with fluorescent tube efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Khanna, V.K. Fundamentals of Solid-State Lighting LEDs, OLEDs, and their Applications in Illumination and Displays, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- D’Andrade, B.W.; Forrest, S.R. White organic light-emitting devices for solid-state lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Muthu, S.; Schuurmans, F.J.P.; Pashley, M.D. Red, green, and blue LEDs for white light illumination. IEEE J. Sel. Top. Quantum Electron. 2002, 8, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; Hu, Z.; Deibert, B.J.; Emge, T.J.; Teat, S.J.; Banerjee, D.; Mussman, B.; Rudd, N.D.; Li, J. Solution processable MOF yellow phosphor with exceptionally high quantum efficiency. J. Am. Chem. Soc. 2014, 136, 16724–16727. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Guo, G.C. Inorganic-organic hybrid white light phosphors. Chem. Commun. 2016, 52, 13194–13204. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Fasol, G. The Blue Laser Diode GaN Based Light Emitters and Lasers; Springer: New York, NY, USA, 1997. [Google Scholar]
- Mosca, M.; Macaluso, R.; Crupi, I. Hybrid inorganic-organic white light emitting diodes. In Polymers for Light-Emitting Devices and Displays; Wiley-Scrivener: Beverly, MA, USA, 2020; pp. 197–262. [Google Scholar]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal-organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Lustig, W.P.; Li, J. Luminescent metal–organic frameworks and coordination polymers as alternative phosphors for energy efficient lighting devices. Coord. Chem. Rev. 2018, 373, 116–147. [Google Scholar] [CrossRef]
- Wang, M.-S.; Guo, G.-C.; Chen, W.-T.; Xu, G.; Zhou, W.-W.; Wu, K.-J.; Huang, J.-S. A White-light-emitting borate-based inorganic–organic hybrid open framework. Angew. Chem. Int. Ed. 2007, 119, 3983–3985. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, H.; Cao, W.; Cui, Y.; Qian, G. Luminescent metal–organic frameworks for white LEDs. Adv. Opt. Mater. 2020, 2020, 2001817. [Google Scholar] [CrossRef]
- Lustig, W.P.; Shen, Z.; Teat, S.J.; Javed, N.; Velasco, E.; O’Carroll, D.M.; Li, J. Rational design of a high-efficiency, multivariate metal-organic framework phosphor for white LED bulbs. Chem. Sci. 2020, 11, 1814–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.Q.; Yin, X.B. Metal-organic frameworks with multiple luminescence emissions: Designs and applications. Acc. Chem. Res. 2020, 53, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Ebrahim, F.M.; Stylianou, K.C. Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coord. Chem. Rev. 2018, 377, 259–306. [Google Scholar] [CrossRef]
- Ryu, U.; Lee, H.S.; Park, K.S.; Choi, K.M. The rules and roles of metal–organic framework in combination with molecular dyes. Polyhedron 2018, 154, 275–294. [Google Scholar] [CrossRef]
- Yu, J.; Cui, Y.; Xu, H.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Confinement of pyridinium hemicyanine dye within an anionic metal-organic framework for two-photon-pumped lasing. Nat. Commun. 2013, 4, 2719. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, Z.; Lin, B.; Hu, X.; Wei, Y.; Zhang, C.; An, B.; Wang, C.; Lin, W. Warm-white-light-emitting diode based on a dye-loaded metal-organic framework for fast white-light communication. ACS Appl. Mater. Interfaces 2017, 9, 35253–35259. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging multifunctional metal-organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273-274, 76–86. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Guo, B.B.; Yin, J.C.; Li, N.; Fu, Z.X.; Han, X.; Xu, J.; Bu, X.H. Recent progress in luminous particle-encapsulated host–guest metal-organic frameworks for optical applications. Adv. Opt. Mater. 2021, 2021, 2100283. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Tang, Y.; Xia, T.; Song, T.; Cui, Y.; Yang, Y.; Qian, G. Efficient energy transfer within dyes encapsulated metal–organic frameworks to achieve high performance white light-emitting diodes. Adv. Opt. Mater. 2018, 6, 1800968. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, Y.; Wang, L.; Lv, Y.; Liu, J.; Zhou, T.L.; Xie, R.J. Color-tunable and high-efficiency dye-encapsulated metal-organic framework composites used for smart white-light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 18910–18917. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Li, Y.; Tsung, C.K.; Li, J. Encapsulation of yellow phosphors into nanocrystalline metal-organic frameworks for blue-excitable white light emission. Chem. Commun. 2019, 55, 10669–10672. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, W.; Yao, L.; Cui, Y.; Yu, Y.; Qian, G. Polyurethane-coated luminescent dye@MOF composites for highly-stable white LEDs. J. Mater. Chem. C 2020, 8, 12308–12313. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Yu, K.; Yu, J.; Zhao, D.; Li, C. Encapsulating carbon quantum dot and organic dye in multi-shell nanostructured MOFs for use in white light-emitting diode. Microporous Mesoporous Mater. 2021, 319, 111062. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Martín, C.; Van der Auweraer, M.; Hofkens, J.; Tan, J.C. Electroluminescent guest@MOF nanoparticles for thin film optoelectronics and solid-state lighting. Adv. Opt. Mater. 2020, 8, 2000670. [Google Scholar] [CrossRef]
- Yin, J.C.; Chang, Z.; Li, N.; He, J.; Fu, Z.X.; Bu, X.H. Efficient regulation of energy transfer in a multicomponent dye-loaded MOF for white-light emission tuning. ACS Appl. Mater. Interfaces 2020, 12, 51589–51597. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, B.; Cui, Y.; Xu, S.; Gong, J. Core–shell structured cyclodextrin metal–organic frameworks with hierarchical dye encapsulation for tunable light emission. Chem. Mater. 2019, 31, 1289–1295. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.Y.; Mo, J.T.; Fu, P.Y.; Zhao, Y.W.; Yin, S.Y.; Jiang, J.J.; Pan, M.; Su, C.Y. White-light emission from dual-way photon energy conversion in a dye-encapsulated metal-organic framework. Angew. Chem. Int. Ed. 2019, 58, 9752–9757. [Google Scholar] [CrossRef]
- Yong, G.; Zhang, X.; She, W. Phosphorescence enhancement of organic dyes by forming β-cyclodextrin inclusion complexes: Color tunable emissive materials. Dye. Pigms. 2013, 97, 65–70. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xing, K.; Li, Y.; Tsung, C.K.; Li, J. Three models to encapsulate multicomponent dyes into nanocrystal pores: A new strategy for generating high-quality white light. J. Am. Chem. Soc. 2019, 141, 14807–14813. [Google Scholar] [CrossRef]
- Song, T.; Zhang, G.; Cui, Y.; Yang, Y.; Qian, G. Encapsulation of coumarin dye within lanthanide MOFs as highly efficient white-light-emitting phosphors for white LEDs. CrystEngComm 2016, 18, 8366–8371. [Google Scholar] [CrossRef]
- Mondal, T.; Bose, S.; Husain, A.; Ghorai, U.K.; Saha, S.K. White light emission from single dye incorporated metal organic framework. Opt. Mater. 2020, 100, 109706. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, H.S.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling metal-organic framework mesopores with TiO2 for CO2 photoreduction. Nature 2020, 586, 549–554. [Google Scholar] [CrossRef]
- Gu, S.F.; Xiong, X.H.; Gong, L.L.; Zhang, H.P.; Xu, Y.; Feng, X.F.; Luo, F. Classified encapsulation of an organic dye and metal-organic complex in different molecular compartments for white-light emission and selective adsorption of C2H2 over CO2. Inorg. Chem. 2021, 60, 8211–8217. [Google Scholar] [CrossRef]
- Cui, Y.; Song, T.; Yu, J.; Yang, Y.; Wang, Z.; Qian, G. Dye encapsulated metal-organic framework for warm-white LED with high color-rendering index. Adv. Funct. Mater. 2015, 25, 4796–4802. [Google Scholar] [CrossRef]
- Xia, Y.-P.; Wang, C.-X.; An, L.-C.; Zhang, D.-S.; Hu, T.-L.; Xu, J.; Chang, Z.; Bu, X.-H. Utilizing an effective framework to dye energy transfer in a carbazole-based metal–organic framework for high performance white light emission tuning. Inorg. Chem. Front. 2018, 5, 2868–2874. [Google Scholar] [CrossRef]
- Wen, Y.; Sheng, T.; Zhu, X.; Zhuo, C.; Su, S.; Li, H.; Hu, S.; Zhu, Q.L.; Wu, X. Introduction of red-green-blue fluorescent dyes into a metal-organic framework for tunable white light emission. Adv. Mater. 2017, 29, 1700778. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, Y.J.; Wang, N.; Zheng, P.; Fu, H.R.; Han, M.L.; Ma, L.F.; Wang, L.Y. Tetraphenylethylene-decorated metal-organic frameworks as energy-transfer platform for the detection of nitro-antibiotics and white-light emission. Inorg. Chem. 2019, 58, 12700–12706. [Google Scholar] [CrossRef]
- Xing, W.; Zhou, H.; Han, J.; Zhou, Y.; Gan, N.; Cuan, J. Dye encapsulation engineering in a tetraphenylethylene-based MOF for tunable white-light emission. J. Colloid Interface Sci. 2021, 604, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Feng, G.; Dong, J.; Lei, S.; Hu, W. Dual-functional porous MOFs with hierarchical guest encapsulation for room-temperature phosphorescence and white-light-emission. Nanoscale 2021, 13, 12466–12474. [Google Scholar] [CrossRef]
- Li, M.; Ren, G.; Yang, W.; Yang, Y.; Yang, W.; Gao, Y.; Qiu, P.; Pan, Q. Dual-emitting piezofluorochromic dye@MOF for white-light generation. Chem. Commun. 2021, 57, 1340–1343. [Google Scholar] [CrossRef]
- Newsome, W.J.; Ayad, S.; Cordova, J.; Reinheimer, E.W.; Campiglia, A.D.; Harper, J.K.; Hanson, K.; Uribe-Romo, F.J. Solid state multicolor emission in substitutional solid solutions of metal-organic frameworks. J. Am. Chem. Soc. 2019, 141, 11298–11303. [Google Scholar] [CrossRef]
- Wu, S.; Ren, D.; Zhou, K.; Xia, H.L.; Liu, X.Y.; Wang, X.; Li, J. Linker engineering toward full-color emission of UiO-68 type metal-organic frameworks. J. Am. Chem. Soc. 2021, 143, 10547–10552. [Google Scholar] [CrossRef]
- Leith, G.A.; Martin, C.R.; Mayers, J.M.; Kittikhunnatham, P.; Larsen, R.W.; Shustova, N.B. Confinement-guided photophysics in MOFs, COFs, and cages. Chem. Soc. Rev. 2021, 50, 4382–4410. [Google Scholar] [CrossRef]






| MOF | Organic Ligand | Ref. |
|---|---|---|
| ZJU-28 |  | [34] |
| Bio-MOF-1 |  | [35] |
| UiO-66 |  | [36] |
| ZIF-8 |  | [36,37,38] |
| NKU-114 |  | [40] |
| LIFM-WZ-6 |  | [42] |
| EuBPT TbBPT |  | [45] |
| [Zn4OL2·xDMF]n |  | [50] |
| HSB-W1 |  | [51] |
| [Cd7(SO4)6(tppe)2] (2DMF·2H2O) [Zn2(npd)2(tppe)](2DMF·3H2O) |  | [54] |
| PCN-128W |  | [55] |
| PCN-921 |  | [56] |
| HNU-49 |  | [57] |
| Dye-Encapsulated MOF Materials | CIE (x,y) | CCT(K) | CRI | QY (%) | Ref. |
|---|---|---|---|---|---|
| ZJU-28⊃Cou-6/R6G/R101 | (0.34, 0.32) | 4446 | 88 | 82.9 | [34] |
| R6G@ZIF-8 | - | - | -- | 63.1 | [36] |
| ZIF-8⊃pm546/pm605/SRh101 | (0.465, 0.413) | 2642 | 85 | - | [37] |
| NKU-114@DSM/AF/9-AA | (0.3402, 0.3365) | 5148 | 85.41 | - | [40] |
| CD-MOF⊃7-HCm@FL@RhB | (0.35, 0.32) | - | - | - | [41] |
| ZJU-28⊃DSM/ AF | (0.34, 0.32) | 5327 | 91 | 17.4 | [49] |
| [Zn4OL2·xDMF]n⊃DCM/C6 | (0.32, 0.31) | 6186 | 91 | 39.4 | [50] |
| HSB-W1⊃DCM/C6a/CBS-127 | (0.31, 0.32) | 6638 | 90 | 26 | [51] |
| DSM@PCN-128W | (0.34, 0.33) | 5525 | 79.1 | 21.2 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Khurshid, A.; Sohail, M.; Qiu, W.; Cao, D.; Su, S.-J. Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes. Nanomaterials 2021, 11, 2761. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102761
Sun Z, Khurshid A, Sohail M, Qiu W, Cao D, Su S-J. Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes. Nanomaterials. 2021; 11(10):2761. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102761
Chicago/Turabian StyleSun, Zhihong, Aaqib Khurshid, Muhammad Sohail, Weidong Qiu, Derong Cao, and Shi-Jian Su. 2021. "Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes" Nanomaterials 11, no. 10: 2761. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102761